 Click on the image to know more about Schultz Dale Reaction
Click on the image to know more about Schultz Dale Reaction
What is Schultz Dale Reaction
Schultz Dale reaction is an anaphylactic reaction produced in vitro during the testing of immunomodulatory drugs
What is the rationale behind Schultz Dale Reaction
It is used to initiate the release of mediators of anaphylaxis like histamine which induce contraction in smooth muscles.
How is Schultz Dale Reaction induced
To induce Schultz Dale reaction, guinea pigs are used generally. The animals are sensitized against egg albumin and three weeks after this, they are sacrificed. Ileum of the sacrificed animals is isolated and the contractility of the isolates is tested
Can we use any other tissues for Schultz Dale reaction?
Yes. Researchers have used lungs, tracheae of guinea pigs and trachea of mice as well.

 , per unit volume of total moist air,
, per unit volume of total moist air, 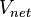 , can be expressed as follows:
, can be expressed as follows:
 in the mixture to the saturated vapor pressure of water
in the mixture to the saturated vapor pressure of water  at a prescribed temperature.
at a prescribed temperature.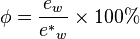
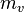 , per unit mass of dry air
, per unit mass of dry air  .
.
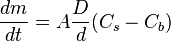
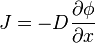
 is the “diffusion flux” [(amount of substance) per unit area per unit time], example
is the “diffusion flux” [(amount of substance) per unit area per unit time], example  .
. 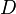 is the diffusion coefficient or diffusivity in dimensions of [length2 time−1], example
is the diffusion coefficient or diffusivity in dimensions of [length2 time−1], example 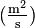
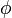 (for ideal mixtures) is the concentration in dimensions of [(amount of substance) length−3], example
(for ideal mixtures) is the concentration in dimensions of [(amount of substance) length−3], example 
 is the position [length], example
is the position [length], example 


 is time [s]
is time [s]
