Contents of the powerpoint on Drug Delivery Technologies for Insoluble Drugs include:
Drug Delivery Technologies for Insoluble Drugs
Resources
All the resources required for pharma students
[PPT] Controlled Release Through Microencapsulation
Contents of the powerpoint on Controlled Release Through Microencapsulation include:
Controlled Release Through Microencapsulation
Introduction
[PPT] Controlled Drug Delivery – Pharmacokinetic Basis
Contents of the powerpoint on Controlled Drug Delivery – Pharmacokinetic Basis include:
Introduction
[PPT] Colon Specific Drug Delivery
Contents of the powerpoint on Colon Specific Drug Delivery include:
Introduction
Advantages and Disadvantanges
Techniques
[PPT] Biopharmaceutic Classfication System (BCS) of Drugs
Contents of the powerpoint on Biopharmaceutic Classfication System (BCS) of Drugs include:
Concept of BCS
Definition
How to Define
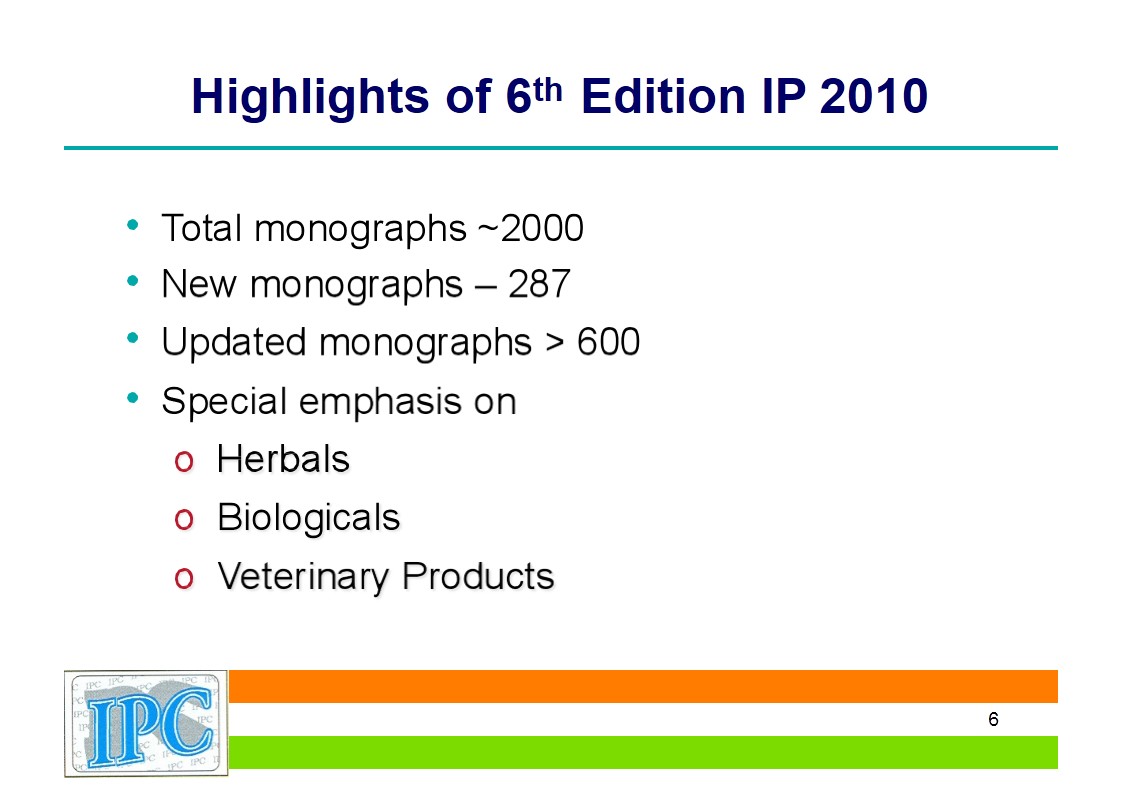
Key Features of Indian Pharmacopoeia 2010 – 6th edition
Download the presentation here
Source: www.who.int
[PPT] The International Pharmacopoeia | WHO – An Overview
[gview file=”http://pharmawiki.in/wp-content/uploads/2013/10/International-Pharmacopeia-A-overview.pps”]
Download the PPT by clicking here
Health ministry to review draft on M Pharm and practice regulation
Health ministry to review draft on M Pharm and practice regulation
In order to set standards in pharmacy education and to bring about an ethics code in pharmacy practice, the health ministry will soon review the draft M Pharm and Pharmacy Practice Regulations framed by the Pharmacy Council of India (PCI).
“The need for an M Pharm regulation has caught the attention of the health ministry considering the challenges pharmacy colleges face in terms of setting quality standards in training. Approval from the government will translate into educational
regulation in post graduate studies with a clear and concrete mandate so that it is properly followed and adhered to by the pharmacy colleges across the country,” PCI president Dr B Suresh said.
“The objective of the M Pharm regulation would be to help the pharmacy institutes address gaps in the delivery of education in a qualitative manner. The regulation once implemented would bring about standards in terms of teacher- student ratio and the infrastructure required.”
M Pharm regulation meant to ensure quality
assurance in post graduate studies will most likely meet stiff resistance from the pharmacy colleges who don’t have funds to meet the requisite standards in terms of staff and infrastructure and hence quality assurance.
Besides this, implementation of the Practice Regulation will benefit the patients in a big way as it will draw an ethics code for the pharmacist to be followed as a part of his/her responsibility to educate patients about the medicines prescribed by the doctor.
The said regulation will bring under its purview all pharmacy professionals working in regulatory bodies, drug stores and academic institutions to cater to patient well being.
In order to further boost the professional development of pharmacists, PCI has also set aside funds for Continuing Education
Programme (CEP) in which an institute or state pharmacy council is entitled to get Rs 25,000 for running the CEP programme having a participation of 100 pharmacists.
These regulations are relevant today going by the upcoming trend of industry driven drug discovery and development. Many of the monographs in pharmacopoeia today for new drugs and devices rely on technology and information from the industry.
Source: CDSCO, New Delhi.
Osmania University B.Pharm Syllabus
Syllabus of Osmania University and its affiliate pharmacy colleges for B.Pharm course.
The list contains subjects, recommended hours along with practicals and also the recommended books for each subject
Board of Studies in Pharmacy
FACULTY OF TECHNOLOGY
OSMANIA UNIVERSITY
RULES AND REGULATIONS FOR B. PHARMACY COURSE
(EFFECTIVE FROM ACADEMIC YEAR 2009 – 2010)
SCHEME OF INSTRUCTION AND EXAMINATION FOR
B. PHARMACY I YEAR
(Effective for the Batch admitted during the Academic Year 2009-10)
| Course No |
Subject |
Periods / week Th. Pr. |
Sess. Marks |
Univ. Exam. Marks |
Duration of Exam (HRS) |
| PYT.1.101 | Anatomy, Physiology and Health Education |
3 — |
30 |
70 |
3 |
| PYT.1.102 | Pharmaceutical Inorganic Chemistry |
3 — |
30 |
70 |
3 |
| PYT.1.103 | Pharmaceutics-I (General and Dispensing Pharmacy) |
3 — |
30 |
70 |
3 |
| PYT.1.104 | Mathematics / Biology |
4/4 — |
30 |
70 |
3 |
| PYT.1.105 | Basic Computer Applications |
3 — |
30 |
70 |
3 |
| PYP.1.106 | Anatomy, Physiology and Health Education Practicals |
— 3 |
25 |
50 |
4 |
| PYP.1.107 | Ph. Inorganic Chemistry Lab |
— 3 |
25 |
50 |
4 |
| PYP.1.108 | Pharmaceutics-I (General & Dispensing Pharmacy) Lab |
— 3 |
25 |
50 |
4 |
| PYP.1.109 | Biology Lab |
— 3 |
25 |
50 |
4 |
| PYP.1.110 | Computer Lab (Basic Comp. Applications) |
— 3 |
25 |
50 |
4 |
|
31 |
275 |
600 |
|
-
ANATOMY, PHYSIOLOGY AND HEALTH EDUCATION
Subject Code : PYT.1.101 Sessional : 30
Periods / Week: 3 Examination
: 70
Nature of Exam: Theory Exam Duration: 3 Hrs
Unit – I
Introduction: Anatomical terms in relation to parts of the body, system and organs. Elementary knowledge of the human skeleton; Tissues of the body – properties and functions of epithelial, connective, muscular, nervous and osteous (bone) tissues; General principles of membrane permeability, diffusion, transport, membrane potentials and action potentials.
Unit – II
Nervous Systems: Neuron, Synapses, ganglion, plexus, physiology of nerve impulse, neurotransmission, reflex arc, central nervous system (parts and functions) and autonomic nervous system.
Cardiovascular System and Blood: Heart, blood vessels, cardiac cycle, circulation, blood pressure and its regulations. Blood (composition and function).
Unit – III
Respiratory System: Gross anatomy of respiratory passages, physiology of respiration, nervous control of respiration, vital capacity, respiratory volume, introduction to terms such as anoxia, hypoxia & dyspnoea.
Digestive System: Gross anatomy of alimentary canal, movements of alimentary canal, gastric secretions and the enzymes involved in digestion.
Endocrine System: Mechanisms of hormonal secretion, Physiological considerations of thyroid, pancreas, pituitary, parathyroid, adrenal glands & gonads; Disorders of hypo & hyper secretion.
Unit – IV
Urinogental System: Various parts, structure and functions of the kidney and urinary tract. Physiology of urine formation, output and factors controlling it.
Physiology of Special Senses: basic anatomy and physiology of the eye (vision), ear (hearing), taste buds (Tongue), nose (smell) and skin (touch and pain).
Unit – V
Health Education (Epidemiology) and Family Planning.
Elementary pathology – Diseased and pathological processes.
Inflammation and repair, Retrograde changes including disturbances of metabolism, circulation like haemorrhage, thrombosis and growth including various tumors (Neoplasms).
Embolism, infarction, Oedema and shock. Nutritional disorder (Vitamin deficiency)
Examination : One question from each unit with internal choice.
Text Books
1.
Principles of anatomy and physiology by Tortora G.J., and N.P. Anagnokokos,
2.
Principles of Anatomy and Physiology by Ross & Wilson.
Reference Books
1.
Human Physiology by C.C. Chatterjee, Medical Allied Agency, India.
2.
Text Book of Medicinal Physiology by A.C. Guyton, W.B. Prism Books Pvt. Ltd.,
-
PHARMACEUTICAL INORGANIC CHEMISTRY
Subject Code : PYT.1.102 Sessional : 30
Periods / Week: 3 Examination
: 70
Nature of Exam: Theory Exam Duration: 3 Hrs
Unit – I
a)
Classification of Inorganic Pharmaceuticals based on their applications, therapeutic classes with example and uses.
b)
Quality control and tests for purity, qualitative tests for anions and cations.
c)
Limit test for Arsenic, heavy metals, Mercury, lead, iron, chloride and Sulphate and Pharmacopoeial Standards.
Note: following units all the compounds are of IP except which are mentioned as BP.
Unit – II
Definition, Preparation, Properties, Assay methods, Limit tests and Uses
a)
Gastro – intestinal agents:
(i)
Acidifiers and Antacids: IP: Dilute hydrochloric acid, sodium acid phosphate, sodium bicarbonate, sodium citrate, Potassium citrate, Aluminum hydroxide gel, Dried Aluminum hydroxide gel, Magnesium oxide (Magnesia), Magnesium-hydroxide mixture, Magnesium carbonate, Magnesium trisilicate, Calcium carbonate.
(ii)
Adsorbents and Related Drugs: Light kaolin, Heavy kaolin, Activated charcoal.
(iii)
Laxatives: Magnesium Sulphate and sodium phosphate.
b) Electrolytes: Sodium, Potassium and Calcium replenishers.
(i) Sodium and Potassium replenishers: Sodium chloride (compound, injection and Ringer solution), Sodium chloride and dextrose injection, Potassium chloride and oral electrolytes.
(ii) Calcium Replenishers: Calcium chloride, Calcium gluconate, Dibasic calcium phosphate.
(c) Acid base Regulators: Sodium bicarbonate, sodium lactate injection, sodium citrate / Potassium citrate, sodium acetate, Ammonium chloride, Ammonium chloride injection.
(d) Dialysis fluids: Haemodialysis fluids and intraperitoneal dialysis fluids.
Unit – III
Definition, Preparation, Properties, Assay methods, Limit tests and Uses
(a)
Mineral Nutrients:
i.
Haematinics: Ferrous Sulphate, Ferrous fumarate, Ferrous gluconate, Ferric ammonium citrate, iron and dextrose injection.
ii.
Metallics: Copper, Manganese and Zinc compounds (zinc chloride);
iii.
Phosphates: Sodium acid phosphate and Sodium phosphate,
iv.
Halogens: Iodine and Iodides or fluorides.
(b) Pharmaceutical aids:
i.
Adsorbents & Absorbents: Activated charcoal, aluminium sulphate, aluminium phosphate.
ii.
Antioxidants: Sodium Sulphite, sodium bisulphate and sodium metabisulphite.
iii.
Desiccants: Silica gel.
iv.
Excipients: Dicalcium & Tricalcium Phosphate, Magnesium stearate, Talc & ppted chalk.
v.
Suspending agents: Bentonite, colloidal silica, aluminium stearate,.
vi.
Colourants: Titanium oxide, ferric oxide
vii.
Solvent and Vehicle: Purified water
Unit – IV
Definition, Preparation, Properties, Assay methods, Limit tests and Uses
i.
Expectorants: of Ammonium chloride, Potassium Iodide.
ii.
Emetics: Potassium antimony tartarate, copper Sulphate, Zinc Sulphate.
iii.
Antidotes: Sodium thiosulphate, sodium thiosulphate injection , sodium nitrite.
iv.
Inhalants: Oxygen, Nitrous oxide, dilute solution of ammonia (BP), Ammonium carbonate (BP).
Unit – V
Definition, Preparation, Properties, Assay methods, Limit tests and Uses
(a)
Topical agents:
i.
Astringents: ZnSO4, Zinc Oxide, Calcium Hydroxide, CuSO4 and Bismuth subcarbonate.
ii.
Topical protectants: Zinc oxide, Calamine, Zinc stearate, Talc, Titanium-dioxide, Heavy kaolin and Light kaolin
iii.
Silicone polymers: Activated Dimethicone.
iv.
Anti infectives: Hydrogen peroxide, Potassium permanganate, Silver Nitrate (Silver protein), Iodine, (solutions, povidone – iodine), boric acid, zinc – undecylenate, Mercury compounds (Yellow mercuric oxide, Ammoniated Mercury). Sulphur, Selenium sulphide.
(b)
Dental products:
i.
Fluorides: Sodium fluoride, Sodium Monofluorophosphate and stannous fluoride.
ii.
Oral antiseptics and Astringents: Hydrogen peroxide, Sodium peroxide (BP), Magnesium peroxide, Zinc peroxide and Mouth washes
iii.
Dentifrices: Calcium carbonate, dibasic calcium phosphate, calcium phosphate, sodium metaphosphate and strontium chloride.
iv.
Cements and Fillers: Zinc oxide.
(c)
Other Medicinal agents:
i.
Internal parasiticides: Sodium Antimony Gluconate
ii.
Anti-neoplastic agents: Cisplatin.
iii.
Sedative-hypnotics: Potassium bromide
iv.
Anti-depressants: Lithium carbonate
v.
Anti-rheumatic agents: Sodium aurothiomalate
vi.
Anti-thyroid agents: Potassium perchlorate
vii.
Diagnostic agent: Barium Sulphate
viii.
Surgical aid: Plaster of Paris
Examination : One question from each unit with internal choice.
Text Books
1. Bentley & Driver’s Text book of Pharmaceutical chemistry Ed: L. M. Atherden, 1983, Oxford University press, Delhi.
2. Inorganic Medicinal & Pharmaceutical chemistry; J. H. Block, F. B. Roche, T.O. Soine, C.V. Wilson, 1986, Varghese publishing house.
3. Inorganic Pharmaceutical chemistry; P. Gundu Rao, Vallabh Prakashan 1995, Delhi
Reference Books
-
1.
Pharmacopoeia; (Indian, British, US and European)
-
2.
Martindale: The Extra Pharmacopoeia; 31st Edn, 1996, The Royal Pharmaceutical Society.
-
3.
Remington Pharmaceutical sciences; 20th Edition Lippincott Williams and Wilkins.
-
4.
Hand Book of Pharmacy & Health care Ed: Robin. J. Haiwan 1990, The Pharm Press, UK
PHARMACEUTICS – I (GENERAL & DISPENSING PHARMACY)
Subject Code : PYT.1.103 Sessional : 30
Periods / Week: 3 Examination : 70
Nature of Exam: Theory Exam Duration: 3 Hrs
Unit – I
Pharmacy profession: Pharmacy as a career, Pharmaceutical Education, Registration as a Pharmacist, Brief introduction to Evolution of Pharmacy, European and American Pharmacy. Pharmacopoeia (IP, BP, USP and International) and other sources, SI and imperial systems, inter conversions. Weighing – selection and care of weights and balances. Sensitivity and minimum weighable quantities.
Pharmaceutical calculations: Calculations of doses, enlarging and reducing recipes; Percentage solutions, alligation, alcohol dilutes and proof spirit.
Unit – II
Prescription: Definition, Parts, sources of errors and care required in dispensing prescriptions, General Dispensing procedures, types of dispensing products. Dispensing of proprietary medicine. Prescription containers, closures and labeling of dispensed products, colors, flavors and sweeteners used in prescription.
Dosage form: Definition, Advantages and limitations of dosage form.
Principles involved and procedures adopted in preparation, labeling and dispensing of typical products (Unit III-IV). Uses of official and other products in common use.
Unit – III
Liquid preparation: Aromatic waters, spirits, solutions, mixtures, syrups, elixirs, suspension, emulsion, lotions, liniments, eye, ear and nasal drops, inhalations, throat paints, gargles, glycerin and collodions.
Unit – IV
Semisolids: Ointments and their bases, creams, jellies, suppositories and their bases, effervescent granules, tablet tritrates, pastilles, lozenges and pills.
Incompatibilities: Physical, Chemical and Therapeutic incompatibilities. Methods of overcoming and handling of incompatible prescriptions.
Unit – V
Tinctures and Extracts: Methods of preparation and uses of Tinctures & Extracts official in IP.
Medicinal Gases: Official medical gases and uses, containers and fitting, handling and storage.
Radio Pharmaceuticals: Preparation, Therapeutic and Diagnostic uses.
Examination : One question from each unit with internal choice.
Text Books
- 1.
Bentley’s text book of pharmaceutics, Rawlkins, 8th edn. ELBS Publishers.
- 2.
Cooper & Gunn’s dispensing for Pharmaceutical students, Carter CBS Publishers, Delhi.
Reference Books
- 1.
Introduction to pharmaceutical dosage forms, HC. Ansel, 5th Edition. 1990.
- 2.
Dispensing of Medication, Ed. E.W. Martin, Mach Publishing Co., Eastern PA.
BIOLOGY
Subject Code : PYT.1.104 Sessional : 30
Periods / Week: 4 Examination : 70
Nature of Exam: Theory Exam Duration: 3 Hrs
Unit – I
Plant Kingdom: Definition and Classification
Plant cells: Its structure, living and non-living inclusions. Different types of plant tissues and their functions, Mitosis and Meiosis.
Morphology and Histology: Roots, Stems, Barks, Woods, Leaf, Flower, Fruit and Seed. Modification: Root, Stem, Leaf and Infloresence.
Unit – II
Plant Taxonomy: Classification, study of the following families with special references to medicinal and economical important plants
a) Apocynaceae b) Solanaceae c) Umbelliferae
d) Leguminosae e) Scrophulariaceae f) Rubiaceae
Unit – III
Plant Physiology: Absorption, transpiration, respiration photosynthesis, basis in DNA replication.
Genetic code and Heredity: Polyploidy, hydridization and mutation.
Unit – IV
The study of animal cell: Animal tissue and cell division, difference between plant cell and animal cell, study of different systems of frog. Histology of liver, kidney, skeletal muscles, smooth muscles, pancrease, intestine and endocrine glands of rabbit.
Unit – V
Morphology and Life History of Human Parasites: Plasmodium, Entamoeba, tapewarm, ascaris, leishmania, anchylostoma and trypanosoma. Life history of Mosquitoes and housefly as agents for spreading diseases.
Examination : One question from each unit with internal choice.
Text books
-
1.
A text book of botany, by A.C. Dutta
-
2.
A text book of biology by Vikram series
MATHEMATICS
Subject Code : PYT.1.104 Sessional : 30
Periods / Week: 4 Examination : 70
Nature of Exam: Theory Exam Duration: 3 Hrs
Unit – I
Logarithms: Logarithm of a real number to an arbitary base, Napierion Base – Theorems on Logarithms – Use of Tables.
Trigonometry: Measurement of angles, Trigonometrical ratios and simple relations connecting the complimentary and supplementary angles, Negetive angles sum and difference of two angles, sine and cosine formulae for multiple angles and half angles.
Unit – II
Differential Calculus: Functions, Limits, Differential coefficient rules, Differentiation of a sum, product and quotient of functions, Differentiation from first principles, Differentiation of implicit, Geometrical, composite and inverse functions, Partial Differentiation, Maxima and Minima.
Unit – III
Integral Calculus: Integration considered as converse of differentiation, simple integrations, standard forms like x dx, Sin (a x) dx, Cos (a x) dx, Sec (a x) dx etc. Methods of substitution, simple example integration by parts. Integration of rational, irrational, trigonometrical functions. Calculations of areas of standard bodies using integration.
Unit – IV
Matrices: Matrices, basic definitions, matrix operations, transpose, adjoint, rank, inverse of a matrix, solution of linear systems of equations, matrix inversion, Gaussian elimination.
Unit – V
Biomathematics: Basic Mathematical Principles that are commonly used in Biological testing, integers, linear and non-linear graphs; 2d Coordinate geometry, Equation of line, circle.
Examination : One question from each unit with internal choice.
Text Books
-
1.
A text book of Mathematics by N.Krishna Murthy, Chand series, Volume- I and II
-
2.
Fundamentals of statistics by D.N. Elhance, Veena Elhance & B.M.Agarwal.
Reference Book
-
1.
Higher Engineering Mathematics by Grewal.
BASIC COMPUTER APPLICATIONS
Subject Code : PYT.1.105 Sessional : 30
Periods / Week: 3 Examination : 70
Nature of Exam: Theory Exam Duration: 3 Hrs
Unit – I Computer Concepts
Evolution, Basic structure and Characteristics of computers; Types of memory chips; Study of various input – out put devices like magnetic tapes, magnetic discs, MICR, OCR, CDROMS etc., Types of printers; Principles of flow charting; Importance of operating systems, detailed study of the operating systems MSDOS , UNIX and WINDOWS; Computer Viruses;
Unit – II Programming In ‘C’ Language
Operators, Expressions, Data input, Output, Control statements like – (IF-ELSE, WHILE DO, FOR, BREAK AND CONTINUE and GOTO) Functions, Library functions, Arrays.
Unit – III Introduction to Ms-Office (Word & Excel)
MS-Word: Basics, working with files, working with text, formatting paragraphs, styles, lists, tables, Graphics, spellings and grammar and page formatting macros, table of contents.
MS-Excel: Basics, Spreadsheets, data types, formulas, Formatting, charts, graphs.
Unit – IV Introduction to Ms-Office (Power Point & Access)
MS-Power Point: Power point basics, Views, Slide control, Apply design, Page setup, Templates, Background, Control, Color Screens, Transitions and animations, working with texts and working with graphics.
MS-Access: Data base concepts, Screen layouts, Creating tables, Data sheet records, table relation ships, Sorting and filtering, Queries, forms, form controls, Sub forms, reports, importing, exporting, linking.
Unit – V Information Infrastructure
Internet and World Wide Web (WWW): Structure and Organization of the WWW, Browsers, Information search in WWW, search engines, Pharmaceutical resources in WWW Types of indexing tools & search strategies; Hyper Text Manuscript Language (HTML) and E-Mail.
Introduction to Structured Query Language (SQL): Overview of SQL Reserved Words; SQL Commands, Comparison for Access and SQL Server; Chemical Database Design & their Tools
Examination : One question from each unit with internal choice.
Text Books
1. Fundamentals of Computers by P.K. Sinha
2. Let Us C by Yashvanth Kanetkar
3. Working in Microsoft Office By Ron Mansfield
4. SQL, PL/SQL The Programming Language of Oracle by Ivan Bayross
Reference Books
1. Programming with ‘C’ by Byron Goltfield- Schum series
2. Computer programming in ‘C’ by Y. Raja Raman
ANATOMY, PHYSIOLOGY AND HEALTH EDUCATION
Subject Code: PYP.1.106 Sessional : 25
Periods/Week: 3 Examination : 50
Nature of Examination: Practical Exam Duration: 4 Hrs
List of Experiments
- 1.
Study of histological slides of different tissues / organs
- 2.
Study of various models, specimens of bones / organs
- 3.
Hematology – blood grouping
- 4.
Hemoglobin content estimation
- 5.
Estimation of bleeding time
- 6.
Estimation of clotting time
- 7.
Determination of RBC count
- 8.
Determination of total WBC count
- 9.
Measurement of blood pressure
- 10.
Measurement of vital capacity
- 11.
Estimation of ESR
Reference Books
-
1.
S.R. Kale and R.R. Kale, Practical Human Anatomy & Physiology, Nirali Prakashan, Pune, 2003.
-
2.
CL Ghai, Text book of Practical Physiology, Jay Pee, New Delhi, 2005.
PHARMACEUTICAL INORGANIC CHEMISTRY (Practicals)
Subject Code: PYP.1.107 Sessional : 25
Periods / Week: 3 Examination : 50
Nature of Examination: Practical Exam Duration: 4 Hrs
List of Experiments
- 1.
Systematic quantitative analysis for inorganic mixtures upto 4 radicals preferably by semi-micro methods.
- 2.
Pharmacopoeial limit test for Chlorides
- 3.
Pharmacopoeial limit test for Sulphates.
- 4.
Pharmacopoeial limit test for lead.
- 5.
Pharmacopoeial limit test for iron.
- 6.
Preparation and purification of Boric acid
- 7.
Preparation and purification of sodium citrate
- 8.
Preparation and purification of potash alum.
- 9.
Preparation and purification of yellow mercuric oxide
- 10.
Preparation and purification of Ammoniated Mercury
Reference Books
-
1.
A.H Beckett and J.B Stenlake, Practical Pharmaceutical Chemistry, 4th Edition, CBS Publications, New Delhi, 2004.
-
2.
G Svehla, Vogel’s Qualitative Inorganic Analysis, 7th Edition, Pearson Education, New Delhi, 2003.
-
3.
G. Devala Rao, Practical Pharmaceutical Inorganic Chemistry, Birla Publications, New Delhi, 2006.
-
4.
K. R. Mahadik and S.H Bhosale, Hand book of Practical Chemistry (Inorganic & Organic), Nirali Prakashan, Pune, 2005.
-
5.
Indian Pharmacopoeia, Controller of Publications, Delhi. 1996.
PHARMACEUTICS – I (GENERAL & DISPENSING PHARMACY)
Subject Code: PYP.1.108 Sessional : 25
Periods / Week: 3 Examination : 50
Nature of Examination: Practical Exam Duration: 4 Hrs
List of Experiments
- 1.
Dispensing Procedures involving pharmaceutical calculation, dosage calculations for pediatric and gaeriatric patients
- 2.
Incompatibility studies in few simple dosage forms.
- 3.
Preparation of Aromatic waters
- 4.
Preparation of spirits
- 5.
Preparation of different types of iodine solution
- 6.
Preparation of cresol soap solution
- 7.
Preparation of compound Sulphur & Calamine lotion
- 8.
Preparation of turpentine liniment
- 9.
Preparation of gargles and throat paint
- 10.
Preparation of sulphur ointment
- 11.
Preparation simple ointment
- 12.
Preparation of whitfield ointment
- 13.
Preparation of non staining iodine ointment
- 14.
Preparation of creams & pastes
- 15.
Preparation of any glycerogelatine based suppository
- 16.
Preparation of Tragacanth jelly
- 17.
Preparation of effervescent granules
- 18.
Preparation of simple syrup
- 19.
Preparation of ear / eye drops
Reference Books
-
1.
C.V.S Subrahmanyam, J. Thimma Setty and G.C. Prabhu Shankar, Laboratory Manual of Pharmaceutics, Vallabh Publications, New Delhi, 2006.
-
2.
R.S Gaud and G.D Gupta, Practical Pharmaceutics,
BIOLOGY
Subject Code: PYP.1.109 Sessional : 25
Periods / Week: 3 Examination : 50
Nature of Examination: Practical Exam Duration: 4 Hrs
List of Experiments
- 1.
Study of plant parts and their modification
- 2.
Study of representative of families – Apocynaceae, Solanaceae, Umbelliferae, Rubiaceae
- 3.
Histology of following crude drugs – Cinchona, Clove, Coriander, Linseed
- 4.
Histological study of different organs through permanent slides
- 5.
Study of various tissues through permanent slides
- 6.
Study of digestive system of frog
- 7.
Study of arterial and venous system of frog
- 8.
Study of male urinogenital system of frog
- 9.
Study of female urinogenital system of frog
- 10.
Study of renal portal system of frog
- 11.
Study of skeletal system of frog
- 12.
Study of spinal nerves system of frog
Reference Books
-
1.
G. Venkateshwar Rao, G. V. Subbaiah and K Sheeba, Intermediate Practical Manual for Botany, Sai Apollo New Century Series, Hyderabad,
-
2.
S. B. Gokhale, C. K. Kokate and D. B. Bidankar, Pharmaceutical Biology, Nirali Prakashan, Pune, 2005.
-
3.
S. B. Gokhale, C. K. Kokate and D. B. Bidankar, Practical Pharmacognosy, Nirali Prakashan, Pune, 2003.
-
4.
S. H. Ansari, Guideline Series for Pharmacognosy, Tata Publishers, New Delhi, 1997.
BASIC COMPUTER APPLICATIONS
Subject Code: PYP.1.110 Sessional : 25
Periods / Week: 3 Examination : 50
Nature of Examination: Practical Exam Duration: 4 Hrs
LIST OF EXPERIMENTS:
01.
Exercised Based on Dos commands
02.
Programming in “C” Language.
03.
Exercises on MS-Office.
04.
Exercises based MS word
05.
Exercises based on MS Excel
06.
Exercises based on MS Access and Power Point.
07.
Programming in SQL
Reference Books
1.sanjay saxena,A first Course computers, vikas publishing House (P) Ltd,N.Delhi,2003
2.Yahhavant Kanetkar,Let Us C , 4th ed, BPB publications,N.Delhi,2002
3.Sanjay saxena , Ms Office 2000 for everone, vikas Publishing House (P) Ltd,N.delhi,2003
Copy Paste from Adobe PDF directly into Word | PDF to Word Converter (Not required!!!)
How many time have u tried copying from a PDF file and pasting into Word?
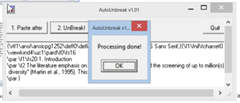
- 1. You did Ctrl+C from a PDF
- 2. And then you hit Ctrl+V into a word document hoping to get this
- 3. But you get this !!!
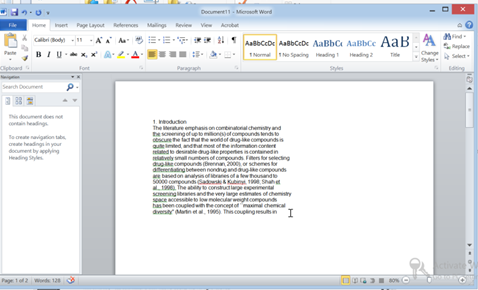
Read on.
- Today we show a simple and cool way of copying from PDF to Word without the need for any PDF to Word converter (Most people use it for this)
i) First of all download this little piece of software called Auto Unbreak.
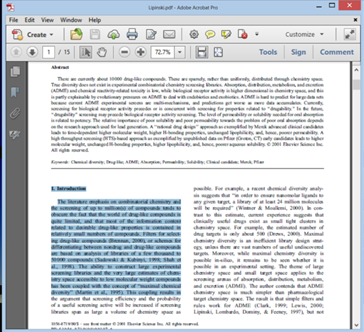
ii) Next Open the File Autounbreak.exe . Copy content from PDF and hit Paste inside the Auto unbreak application we just downloaded
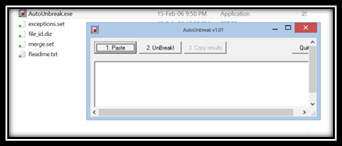
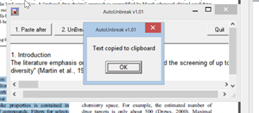
- iii) Next step hit the unbreak button
- iv) Press Ok and then select Copy button
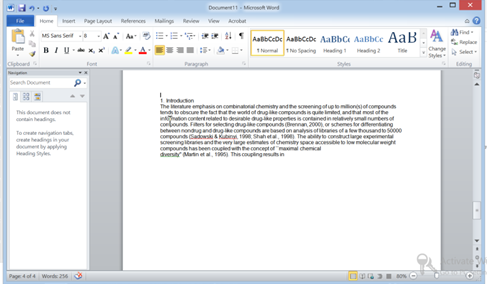
- v) Then Open Word and Now just hit Ctrl+V
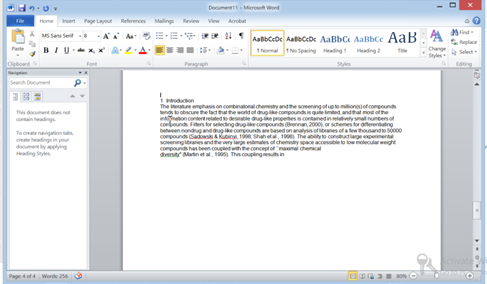
Congratulations its pasted perfectly…
Simple isn’t it? Don’t blame me if you didn’t come across this tool earlier. Share it with your friends . Save your time and others’ too
Note: A small appeal to all those who read my articles. Writing articles requires a lot deal of time (like this one). Although it seems simple a typical post takes around 3-4 hours to work. Pharmawiki as you can see is free of Ads and hence you know that we do not get any financial benefit . The only thing that keeps me going is your feedback. So please share posts with your friends, post in comments if you have any trouble and use the contact us form to bring our attention to a particular topic. We will be more than happy to help.