[gview file=”http://pharmawiki.in/wp-content/uploads/2012/06/Namereactions.pdf”]
GPAT NIPER Syllabus Notification Registration Qualifying marks Result Admit card
Lets see what is GPAT & NIPER.
GPAT -GRADUATE PHARMACY APTITUDE TEST (GPAT)
GPAT
GRADUATE PHARMACY APTITUDE TEST (GPAT) is a national level entrance exam conducted by All India Council for Technical Education (AICTE) every year as per the directions of Ministry of Human Resource Development (MHRD), Government of India. This test facilitates institutions to select suitable Pharmacy graduates for admission into the Master’s (M.Pharm) program. The GPAT is a three hour computer based online test which is conducted in a single session. The GPAT score is accepted by all AICTE-Approved Institutions/University Departments/Constituent Colleges/Affiliated Colleges. A few scholarships and other financial assistance in the field of Pharmacy are also given on the basis of the GPAT score. The GPAT 2018 will be conducted on 21st January, 2018.
National Institute of Pharmaceutical Education and Research (NIPER)
National Institute of Pharmaceutical Education and Research (NIPER) is the first national level institute in pharmaceutical sciences with a proclaimed objective of becoming a centre of excellence for advanced studies and research in pharmaceutical sciences. The Government of India has declared NIPER as an ‘Institute of National Importance’. It is an autonomous body set up under the aegis of Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India. The Institute is conceived to provide leadership in pharmaceutical sciences and related areas not only within the country, but also to the countries in South East Asia, South Asia and Africa. NIPER is a member of Association of Indian Universities and Association of Commonwealth Universities.
In one sense there are three parts of our lives. Past, present and future. How each of these is viewed has huge consequences.
The past:If one looks back at the past for anything other than experience, the past can paralyze today. Many times our memories of events, and results from those events will seem relevant to a situation we might be facing today when in reality today’s situation is totally different. If we treat the current event like the remembered event, we probably have not addressed it correctly. Each problem, challenge, and situation today requires a fresh new outlook and solution. To be sure, we can use look at past experience for suggestions, but today’s solution will always be different.
The future:One of the biggest blocks to living a full life is to spend an inordinate amount of time worrying about the future. Many people fear an unknown future which makes it impossible to live today. This is especially true for those in business for themselves. When one is beginning a business, with no revenue, no customers and startup costs depleting financial reserves, fear of the future can be overwhelming. In fact, this fear can easily incapacitate one to the point that nothing at all gets done due to the ‘what if’ scenarios developing from visions of a horrible failure sure to come.
What if – I fail?
What if – no customers show up?
What if – I run out of money?
What if?
The cure for fear of the future is to keep reminding one’s Self to live in the present moment as much as possible. To fully focus on what is happening right now is challenging. When bank accounts are shrinking, customers and clients are slow to arrive, and the gut reaction is to extrapolate the current conditions into the future, fear has an opening and will take full advantage!
In the time it takes to think through the process and create this fabricated future, fear steps into a life and plays havoc. The truly sad part is many will create that fabricated future because they already believe it. They will unconsciously make choices to bring that future to reality.
The present:The present is a daily, minute by minute gift to us from the Universe. The present needs to be appreciated, caressed and cared for.
It is possible to break this vicious cycle of fear based on unreal projections of things that might happen..
Understand the process. Be aware when it is happening to you.
Create the future you want in your mind. (Visualization)
Make a plan to achieve your future. What would you have to do in order to make it reality?
Work your plan.
Measure your progress and adapt your plan.
Have faith in your Self and the Universal laws that will come to your aid.
So work hard and Get GPAT NIPER
gpat 2018 syllabus
aicte gpat 2018
gpat registration 2018
gpat qualifying marks
gpat 2018 result
gpat admit card 2018
gpat 2019
gpat syllabus
niper admission 2018
niper login
niper ahmedabad
niper branches
niper jee
niper syllabus
niper jee 2018
niper 2018
National Institute of Pharmaceutical Education and Research, Mohali
Public university in Punjab
Indian public Pharmacy research university, and a part of the seven schools, under India’s Ministry of Chemicals and Fertilizers. Wikipedia
How many times can I attempt the GPAT?
What are the advantages of the GPAT?
What are benefits of cracking GPAT exam?
How to prepare for my gpat (pharmacy) exam
niper login
niper official website
niper exam eligibility
niper jee 2018
niper 2018 syllabus
niper exam syllabus pdf
niper hyderabad
niper notification
New features of Indian Pharmacopoeia (IP – 2010)
[gview file=”http://pharmawiki.in/wp-content/uploads/2012/06/New-features-of-IP-2010.pdf”]
List of Test Organisms for Various Antibiotics-Made easy
[gview file=”http://pharmawiki.in/wp-content/uploads/2012/06/Test-Organisms.pdf”]
NIPER JEE – Mnemonics for drugs
Mnemonics for easily remembering drugs and their adverse effects
Steroids side effects: BECLOMETHASONE:
- Buffalo hump
- Easy bruising
- Cataracts
- Larger appetite
- Obesity
- Moonface
- Euphoria
- Thin arms & legs
- Hypertension/ Hyperglycaemia
- Avascular necrosis of femoral head
- Skin thinning
- Osteoporosis
- Negative nitrogen balance
- Emotional liability
For more drugs Download pdf here
[gview file=”http://pharmawiki.in/wp-content/uploads/2012/06/Mnemonics-in-Tabular-form.pdf”]
Test Organisms for Microbiological Assay of Antibiotics
|
Test Organisms for Microbiological Assay of Antibiotics |
|||
| Antibiotic | Test Organism | ATCC No. | NCTC No. (NCIB No.) |
| Amikacin | Staphylococcus aureus |
29737 |
7447 |
| Amphotericin B | Saccharomyces cerevisiae |
9763 |
10716 |
| Bacitracin | Micrococcus luteus |
10240 |
7743 |
| Bleomycin | Mycobacterium smegmatis |
607 |
|
| Carbenicillin | Pseudomonas aeruginosa |
25619 |
|
| Doxycycline | Staphylococcus aureus |
29737 |
7447 |
| Erythromycin | Micrococcus luteus |
9341 |
(8553) |
| Framycetin | Bacillus pumilus |
14884 |
8241 |
| Bacillus subtilis |
6633 |
8236,10400 | |
| Gentamicin | Staphylococcus epidermidis |
12228 |
(8553) |
| Kanamycin sulphate | Bacillus pumilus |
14884 |
8241 |
| Staphylococcus aureus |
29737 |
7447 |
|
| Kanamycin B | Bacillus subtilis |
6633 |
8236 |
| Neomycin | Staphylococcus epidermids |
12228 |
(8553) |
| Novobiocin | Staphylococcus epidermids |
12228 |
(8553) |
| Nystatin | SaccharomycesCerevisiae |
2601 |
10716 |
| Oxytetracycline | Bacillus cereus var, mycoides |
11778 |
10320 |
| Staphylococcus aureus |
29737 |
7447 |
|
| Polymyxin B | Bordetella bronchiseptica |
4617 |
8344 |
| Rifampicin | Bacillus subtilis |
6633 |
8236 |
| Streptomycin | Bacillus subtilis |
6633 |
8236 |
| Klebsiella pnumoniae |
10031 |
(9111) |
|
| Tetracycline | Bacillus Cereus |
11778 |
10320 |
NIPER JEE 2012 (syllabus) updated: 07/06/12 !!!
NIPER JEE Syllabus
(There is NO OFFICIAL syllabus provided for NIPER-JEE exam, but things outside this are rarely asked)
Natural Products:
- In natural products more stress should be given on phytochemistry part rather than biological aspects but you should know about biological sources and chemical constituents of important ones.
- Methods of extraction, isolation and characterization of natural products. Various separation techniques used for isolation of natural products.
- Biosynthetic pathways.
- Primary metabolites, their examples.
- Secondary metabolites, various classes of secondary metabolites – Here most important part is chemistry of these classes. (e.g. Alkaloids, glycosides, tannins, lignans, saponins, lipids, flavonoids, coumarins, anthocyanidines etc).
- Important therapeutic classes: antidiabetics, hepatoprotectives, immmunomodulators, neutraceuticals, natural products for gynecological disorders, anti-cancer, anti-viral (mainly anti-HIV), adaptogens etc. dietary antioxidants, marine natural products, plant growth regulators.
- Standardization of natural products.
- What is difference between natural products and pharmacognosy?
- Some knowledge about types and preparation of ayurvedic formulations like asava, arista etc.
- Stereochemistry and spectroscopy applied to some phytochemical constituents/ pure natural products- NMR, IR. Stereochemistry: Fischer, Sawhorse and Newman projection formulae.
References:
For various therapeutic classes: Trease and Evans
For spectroscopy: Silverstein, Pavia, Kemp
Download full syllabus here
[gview file=”http://pharmawiki.in/wp-content/uploads/2012/06/NIPER-JEE-Syllabus-2012-Updated.pdf”]
FDA Approved and Recalled drugs from Jan 2012 to May 2012
FDA-Approved Drugs in 2012
Dermatology/Plastic Surgery
Sklice (ivermectin) lotion; Sanofi Pasteur; For the treatment of head lice, Approved February 2012
Endocrinology
Korlym (mifepristone); Corcept Therapeutics; For the control of hyperglycemia in adults with endogenous Cushing’s syndrome, Approved February 2012
Hematology
Elelyso (taliglucerasealfa); Pfizer Inc; For the treatment of Gaucher disease, Approved May 2012
Omontys (peginesatide); Affymax; For the treatment of anemia due to chronic kidney disease, Approved March 2012
Musculoskeletal
Stendra (avanafil); Vivus; For the treatment of erectile dysfunction, Approved April 2012
Oncology
Afinitor (everolimus); Novartis Pharmaceuticals Corporation; For the treatment of renal angiomyolipoma associated with tuberous sclerosis complex, Approved April 2012
Erivedge (vismodegib); Genentech; For the treatment of basal cell carcinoma, Approved January 2012
Inlyta (axitinib); Pfizer; For the treatment of advanced renal cell carcinoma, Approved January 2012
[gview file=”http://pharmawiki.in/wp-content/uploads/2012/06/FDA-2012-Newly-approved-drugs-imp-for-niper-jee.pdf”]
FDA Approved and Recalled drugs from May 2011 to Dec 2011
FDA-Approved Drugs in 2011
Cardiology/Vascular Diseases
Brilinta (ticagrelor); AstraZeneca; For the reduction of thrombotic events in patients with acute coronary syndrome, Approved July 2011
Edarbyclor (azilsartanmedoxomilandchlorthalidone); Takeda Pharmaceutical; For the treatment of hypertension, Approved December of 2011
Xarelto (rivaroxaban); Janssen Pharmaceuticals; For the reduction in the risk of stroke and systemic embolism resulting from atrial fibrillation, Approved November 2011
Dermatology/Plastic Surgery
Firazyr (icatibant); Shire; For the treatment of acute attacks of hereditary angioedema, Approved August of 2011
laViv (azficel–T); Fibrocell Science; For the improvement of nasolabial fold wrinkles in adults, Approved June 2011
Endocrinology
Tradjenta (linagliptin); Boehringer Ingelheim; For the treatment of type II diabetes, Approved May 2011
Gastroenterology
Dificid (fidaxomicin); Optimer Pharmaceuticals; For the treatment of Clostridium difficile-associated diarrhea, Approved May 2011
Incivek (telaprevir); Vertex; For the treatment of genotype 1 chronic hepatitis C, Approved May 2011
Victrelis (boceprevir); Merck; For the treatment of chronic hepatitis C genotype 1, Approved May 2011
for more click here
[gview file=”http://pharmawiki.in/wp-content/uploads/2012/06/FDA-2011-Newly-approved-drugs-imp-for-niper-2012.pdf”]
NIPER JEE 2012 Important dates, Admission, Application
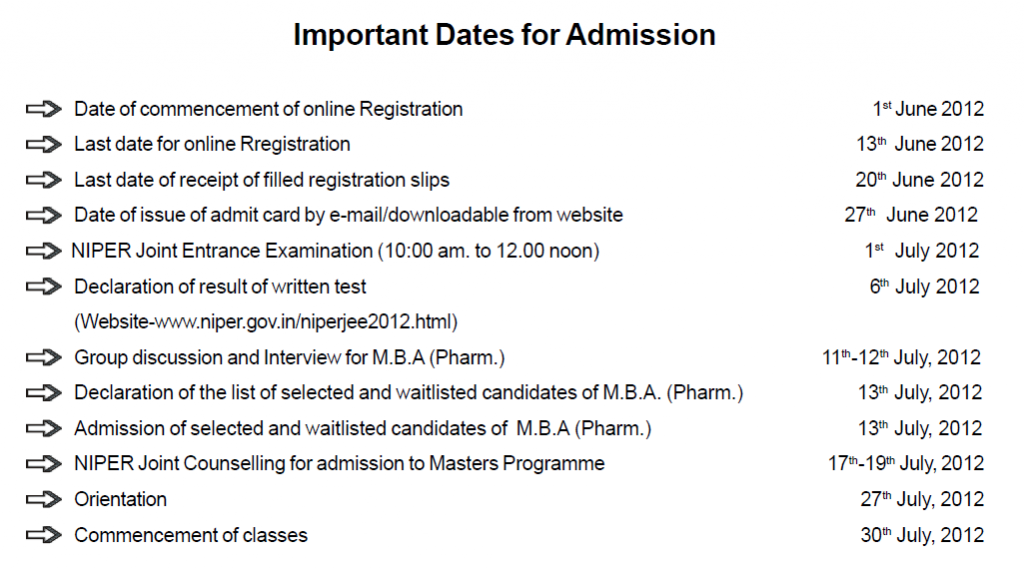
Important Dates for Admission
Date of commencement of online Registration———1st June 2012
Last date for online Rregistration
13th June 2012
Last date of receipt of filled registration slips
20th June 2012
Date of issue of admit card by e-mail/downloadable from website
27th June 2012
NIPER Joint Entrance Examination (10:00 am. to 12.00 noon)
1st July 2012
Declaration of result of written test
6th July 2012 (Website-www.niper.gov.in/niperjee2012.html)
Group discussion and Interview for M.B.A (Pharm.)
11th-12th July, 2012
Declaration of the list of selected and waitlisted candidates of M.B.A. (Pharm.)
13th July, 2012
Admission of selected and waitlisted candidates of M.B.A (Pharm.)
13th July, 2012
NIPER Joint Counselling for admission to Masters Programme
17th-19th July, 2012
Orientation
27th July, 2012
Commencement of classes
30th July, 2012
GPAT-2012 results declared
[pageview url=”http://14.139.121.104:8080/jsp/ResultView.jsp” title=”GPAT 2012 result from www.gpat.in”]


