Heat distribution of an empty chamber: Thermocouples should be situated according to a specific predetermined pattern. Repeatability of temperature attainment and identification of the cold spot can be achieved if the temperature range is ±15°C at all monitored locations. Heat-distribution studies can also be conducted as a function of variable airflow rates
Constraining the temperature range across the loaded chamber has the goal of assuring sterilization is attained across the load, without over-processing of the filled containers.
Performance Qualification Protocol (PQP) – Steam/Air Cycle- Heat distribution
Performance Qualification Protocol (PQP) for Steam/Air Cycle in the Production Steam Steriliser (Autoclave)
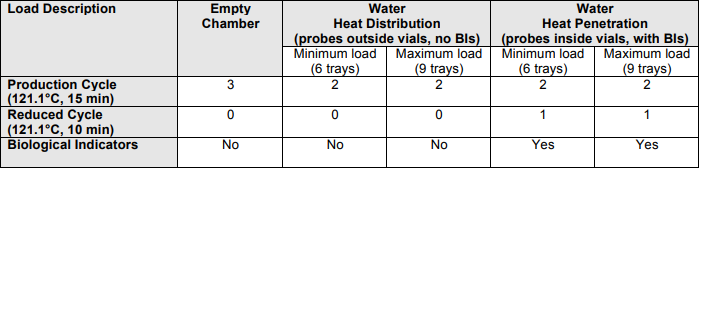
The Production Steam Steriliser (autoclave), shall be used for sterilising aseptically-filled vials of selected products. To qualify the performance of the Fedegari Steam Steriliser (Autoclave) as part of a change control qualification study (refer to CR-14- xxxx “Recommence Manufacture of xxxx Acid Injection 15 mg in 1 mL ) This Performance Qualification shall be limited to demonstrating consistency and efficacy of the steam/air sterilisation cycle, using 1mL water filled into 2 mL vials. Equivalence for xxx Injection 15 mg in 1 mL product, which has been aseptically filled into 2 mL vials, shall be demonstrated in the subsequent Process Validation Study (see PQ protocol kkk). This protocol has been prepared with reference to the following regulatory guidelines: The Performance Qualification study (PQP kkk ) for the autoclave equipment, included heat distribution studies for a porous load cycle only. This document shall include heat distribution studies for the steam/air sterilisation cycle, as part of the process development for the terminal sterilisation of filled vials. The objective of this Performance Qualification, is to verify that the sterilising autoclave consistently provides the required sterility assurance, when operated under normal conditions, using standard minimum and maximum loading patterns and the specified settings: The production cycle registered for Folic Acid product is 121.1 o C for fifteen (15) minutes, to provide a minimum Fo = 8 min. The standard loading patterns shall be as follows: Minimum load Six (6) trays 2 mL vials, 340 vials per tray, across two autoclave shelves Maximum Load Nine (9) trays 2 mL vials, 340 vials per tray, across three autoclave shelves A reduced cycle shall also be run, for the standard production load patterns, to demonstrate that sub-optimal conditions also yield an acceptable level of sterility assurance. This shall be achieved by changing the timetemperature combination for the standard production load patterns to 121.1 o C, for ten (10) minutes, which is 66% of the registered sterilising condition of 121.1o C for 15 minutes.
Process Description
Sterilisation shall be by the moist heat process, using saturated steam, where F0 > 8 DT (see below). A steam-air mixture is used to control chamber pressure and assist in pressure equalisation between chamber and vials, particularly during the cool-down phase. In-process controls for the sterilisation phase of the cycle shall be temperature TE1 in the liquid product and sterilisation phase hold time. Temperature (TE 8 on the auxilliary heating device) and chamber pressure (TP01) are also required to control heating and forced cooling phases of the cycle. For the purposes of the PQ, sterilisation phase temperature and hold time data shall be processed to demonstrate that the physical characteristics of the cycle in terms of accumulated lethality (Fphys) exceed the minimum cycle design criteria (Fo), where: Fo > 8 DT for DTref = D121.1oC = 1.0 minutes and Fphys = Δt ∑10(T-Tref)/z Reference: BP Appendix XVIII “Methods of Sterilisation” and registered particulars, where Fo > 8 min is required. Biological Indicators shall be selected, to demonstrate the survival probability of a non-sterile unit (PNSU) ≤ 10–6 for both the normal production cycle (F0 ≥ 15) and a reduced cycle (F0 ≥10).
Performance Qualification Tests
Tests to be conducted and acceptance criteria are defined in the attached Performance Qualification Test Sheets.
Tests to be performed:
1 Test Instrument Calibration
2 Vacuum Leak Rate Test
3 Heat Distribution Test (empty chamber temperature mapping)
4 Heat Distribution Test (loaded chamber temperature mapping)
5 Heat Penetration studies for:
Production cycle standard loads (121.1 o
C for 15 minutes, two consecutive cycles) and for “Reduced”
cycle standard loads (121.1 o
C for 10 minutes, one cycle)
6 Biological challenge testing for standard and reduced cycle loads
Click here for complete detail
Performance Qualification Protocol (PQP) for Steam/Air Cycle in the Production Steam Steriliser (Autoclave)heat distribution Performance Qualification Protocol for Steam Air Cycle in the Production Steam Steriliser Autoclave