M pharm Pharmaceutics Notes is made available for you guys on this day on the topic EVALUATION OF COLON-SPECIFIC DRUG DELIVERY SYSTEMS. Please have a look at it.
EVALUATION OF COLON-SPECIFIC DRUG DELIVEY SYSTEMS
Various in vitro and in vivo evaluation techniques have been developed and proposed to test the performance and stability of colon-specific drug delivery systems.
-
In vitro dissolution testing
Dissolution testing has been an integral component in pharmaceutical research and development of solid dosage forms. It provides decisive information on formulation selection, the critical processing variables, in vitro/in vivo correlation and quality assurance during clinical manufacturing. In order to provide this information, dissolution testing should be conducted in physiochemically and hydrodynamically defined conditions to simulate the environment that the dosage form encounters in the GI tract. Currently, four dissolution apparatus are recommended in the USP to accommodate different actives and dosage forms: basket method, paddle method, Bio-Dis method and flow-through cell method. However, certain constraints associated with USP dissolution methods were recognized, especially in the dissolution evaluation of complex controlled release drug delivery systems for oral application, and modification of USP dissolution methods to evaluate such delivery systems was deemed necessary (Pillay and Fassihi, 1999). For in vitro evaluation of colon-specific drug delivery systems, the ideal dissolution testing should closely mimic the in vivo conditions with regard to pH, bacteria, types of enzymes, enzymatic activity, fluid volume and mixing intensity.
-
Conventional dissolution testing
Dissolution testing of colon delivery systems with the conventional basket method has usually been conducted in different buffers for different periods of time to simulate the GI tract pH and transit time that the colon-specific delivery system might encounter in vivo (Rudolph et al., 2001). For example, Takeuchi et al., (2000) assessed the dissolution of spray-dried lactose composite particles containing alginate-chitosan complex as a compression coating in pH 1.2 and 6.8 buffers. Results indicated that such dry-coating showed excellent acid-resistance and prolonged induction periods for drug release.
USP Dissolution Apparatus III (reciprocating cylinder) was employed to assess in vitro performance of guar-based colonic formulations. Because of the unique setup of dissolution apparatus III (i.e. the dissolution tubes can be programmed to move along successive rows of vessels), drug release can be evaluated in different medium successively. Wong et al., (1997) evaluated several guar-based colonic formulations using apparatus III in simulated gastric fluid (pH 1.2), simulated intestinal fluid (pH 7.5) and simulated colonic fluids containing galactomannanase. As expected, when compared with drug release in simulated gastric and intestinal fluids, results showed that drug release was accelerated in the colonic fluid due to the presence of the galactomannanase that could hydrolyze the guar gum.
Despite the simplicity and convenience, conventional dissolution testing primarily provides essential information on the processing specifications of a colon-specific delivery system rather than on the validity of the system design. For those delivery systems triggered by bacteria in the colon, the conventional dissolution testing appears unlikely to be predictive of in vivo performance. Additional factors that make conventional dissolution testing of colon-specific drug delivery systems less predictive of its in vivo performance are scarcity of fluid and reduced motility in the colon. One function of colon is to absorb water (Debongnie and Phillips, 1978) and thus condense the luminal contents into semisolids. This would influence the drug release from the system and diffusion within luminal contents.
-
Alternative method for evaluation of colon-specific delivery system in vitro
To overcome the limitation of conventional dissolution testing for evaluating the performance of colon-specific delivery systems triggered by colon-specific bacteria, animal caecal contents including rats (Rubinstein et al., 1993), rabbits (Larsen et al., 1989), and pigs (Larsen et al., 1989) have been utilized as alternative dissolution medium. Because of the similarity of human and rodent colonic microflora, predominantly comprising Bifidobacterium, Bacteroides and Lactobacillus, rat caecal contents were more commonly used in the dissolution studies. Rat caecal contents were usually prepared immediately prior to the initiation of drug release study due to the
anaerobic nature of the cecum. Rats were anaesthetized and the cecum was exteriorized for collection of the contents. The caecal contents were diluted with phosphate-buffered saline (PBS, pH 7) to obtain an appropriate concentration for release study. This step was conducted under CO2 or nitrogen to maintain an anaerobic environment. The drug release studies were generally carried out in sealed glass vials at 37 0C for a defined period of time. Samples were withdrawn at different intervals for analysis (Rubinstein et al., 1992, 1993; Yang et al., 2001).
In the present in vitro study, the volume of dissolution fluid, containing rat caecal contents, was only 100 ml in order to simulate the fluid volume of the colon. Apparatus 2 is not suitable since the wider paddle blade (diameter 75 mm) can not be dipped in the dissolution fluid contained in the beaker (diameter 55 mm).
USP apparatus 3 was used for the evaluation of guar gum formulations meant for colonic drug delivery (Wong et al., 1997). In this study the authors used water soluble enzyme, galactomannase, at a concentration of 0.01 mg/ml. The level of polysaccharidases in 4 g of rat caecal contents used in the present study, though not estimated, may be far less than what was used by Wong et al., (1997). Hence, it is necessary that the guar gum formulations be continuously in contact with the dissolution fluid for better access to the caecal enzymes. This could be achieved by the use of USP apparatus 1. Moreover, the use of USP apparatus 3 also results in settling of the rat caecal contents in the bottom of the vessel. The maintenance of an anaerobic environment in USP apparatus 3 may also be problematic. Because of these reasons, USP apparatus 1 with slight modifications was used in the present study to evaluate guar gum as a carrier in the form of compression coat for colon-specific drug delivery. Further, earlier workers (Ashford et al., 1993b, Krishnaiah et al., 1998) also used apparatus 1 for the evaluation of colonic delivery systems.
-
In vivo evaluation of colon-specific drug delivery systems
As in other controlled release delivery systems, the successful development of a colon-specific drug delivery system is ultimately determined by its ability to achieve colon-specific drug release and thus exert the intended therapeutic effect. When the system design is conceived and prototype formulation with acceptable in vitro characteristics is obtained, in vivo studies are usually conducted to evaluate the site specificity of drug release and to obtain relevant pharmacokinetics information of the delivery system. Although animal models have obvious advantages in assessing colon-specific drug delivery systems, human subjects are increasingly utilized for evaluation of this type of delivery systems with visualization techniques such as γ-scintigraphy imaging.
-
Animal studies
Different animals have been used to evaluate the performance of colon-specific drug delivery systems, such as rats (Van den Mooter et al., 1995; Tozaki et al., 2001), pigs (Friend et al., 1991; Gardner et al., 1996), and dogs (Yang et al., 2001). To closely simulate the human physiological environment of the colon, the selection of an appropriate animal model for evaluating a colon-specific delivery system depends on its triggering mechanism and system design. For instance, guinea pigs have comparable glycosidase and glucuronidase activities in the colon and similar digestive anatomy and physiology to that of human (Hawksworth et al., 1971), so they are more suitable in evaluating glucoside and glucuronate conjugated prodrugs intended for colon delivery.
Friend et al., (1991) evaluated the therapeutic efficacy of dexamethasone-β-D-glucoside with dexamethasone in guinea pigs with experimentally induced IBD (Friend et al., 1991). Even though guinea pig is the preferred animal model to investigate the in vivo performance of certain colon specific delivery systems, it is difficult to administer the delivery system orally.
Rats were also used to evaluate colon-specific drug delivery systems based on azo-polymers or prodrugs containing azo bonds because the distribution of azoreductase activity in GI tract is similar between rats and human subjects (Renwick., 1982).
Another animal commonly used to evaluate oral controlled release delivery systems is the dog (Renwick, 1982). The in vivo performance of CODES™ was evaluated in beagle dogs using acetaminophen as a model drug and lactulose as the matrix-forming excipient in the core tablet (Yang et al., 2001).
It is well recognized that significant differences exist between human subjects and commonly used laboratory animals in GI tract anatomy and physiology, including GI transit time, pH, distribution of enzyme activity, population of bacteria, etc. Therefore, the data obtained from animal models should be interpreted with caution.
-
Gamma-Scintigraphy
In most cases, conventional pharmacokinetic evaluation may not generate sufficient information to elucidate the intended rationale of system design. γ-Scintigraphy is an imaging modality, which enables the in vivo performance of drug delivery systems to be visualized under normal physiological conditions in a non-invasive manner. Through γ-scintigraphy imaging, the following information regarding the performance of a colon-specific delivery system within human GI tract can be obtained: the location as a function of time, the time and location of both initial and complete system disintegration, the extent of dispersion, the colon arrival time, stomach residence and small intestine transit times.
The in vivo performance of the colonic delivery system based on pectin and galactomannan coating was also evaluated in healthy human subjects with γ-scintigraphy together with conventional pharmacokinetic analysis using nifedipine as a model drug (Pai et al., 2000). Overall, γ-scintigraphic results demonstrated that it took 5.44 h for the tablets to reach the ascending colon in 92% of 12 subjects. Upon arrival in the ascending colon, approximately additional 1 h was required to initiate the tablet disintegration. The mean plasma concentration of nifedipine was negligible for more than 5 h post-dose, and then increased rapidly. The pharmacokinetic profile exhibited a good correlation with the scintigraphic results. In essence, γ-scintigraphic evaluation of a colon-specific drug delivery system provides ‘proof of concept’, i.e. visualization of system disintegration event and ascertainment of disintegration location in the GI tract.
M pharma pharmaceutics notes – evaluation of colon specific drug delivery systems PDF doc M pharm Pharmaceutics Notes EVALUATION OF COLON-SPECIFIC DRUG DELIVEY SYSTEMS
-
Roentgenography
The inclusion of a radio-opaque material into a solid dosage form enables it to be visualized by the use of X-rays. By incorporating barium sulphate into a pharmaceutical dosage form, it is possible to follow the movement, location and the integrity of the dosage form after oral administration by placing the subject under fluoroscope and taking series of X-rays at various time points. This technique was used by Dew et al., (1982) to evaluate a capsule dosage form coated with Eudragit S to deliver orally ingested drugs to the colon using barium sulphate as a radio- opaque material.
Table 4. Marketed colon specific drug delivery systems
| Drug | Trade Name | Coating Polymers |
| Mesalazine | claversa®
Asacolitin Mesazal Asacol |
Eudragit® L100
Eudragit® S Eudragit® L100 Eudragit® S |
| Budesonide | Entrocort®
Budenofalk® Targit® |
Eudragit® L100-55
Eudragit® S Coated Starch Capsule |
| Sulfasalazine | Azulfidine
Colo-Pleon |
Cellulose acetate phthalate
Eudragit® L100-55 |
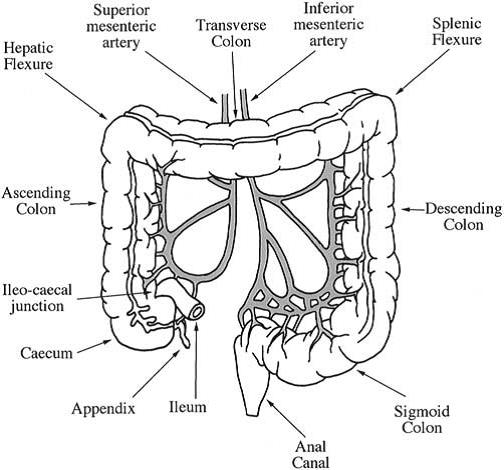
Colon – ANOTOMY & PHYSIOLOGY OF COLON Functions Pharmacology Notes
B Pharmacy M pharmacy Study Material Pharmacology Notes PDF DRUGS SUITABLE FOR COLONIC DRUG DELIVERY


 Click here to download
Click here to download