Contents of the powerpoint on Aseptic Processing Operation include:
Introduction to aseptic processing,
Aseptic Processing vs. Terminal Sterilization
contamination: Sources and control,
Microbial environmental monitoring
Microbiological testing of air and water
Characterization of aseptic process,
Media and incubation conditions.
Conclusion
References
Download the powerpoint by liking us on Facebook
[like-gate][/like-gate]
[button url=”http://pharmawiki.in/?attachment_id=3406″ style=”glass” background=”#1782f9″ color=”#ffffff” size=”4″ center=”yes” icon=”icon: download”]Download PPT here[/button]
You can also download the PDF onAseptic Processing Operation by clicking here
[button url=”http://pharmawiki.in/?attachment_id=3406″ style=”glass” background=”#1782f9″ color=”#ffffff” size=”4″ center=”yes” icon=”icon: download”]Download PDF here[/button]
Introduction to Aseptic Processing
Aseptic processing is a critical technique in pharmaceutical and biotechnology industries that ensures the sterile manufacturing of products such as injectable drugs, biologics, and sterile medical devices. It involves the handling of sterile materials in a controlled environment to prevent microbial contamination and maintain product integrity. This article delves into various aspects of aseptic processing, including its key principles and applications.
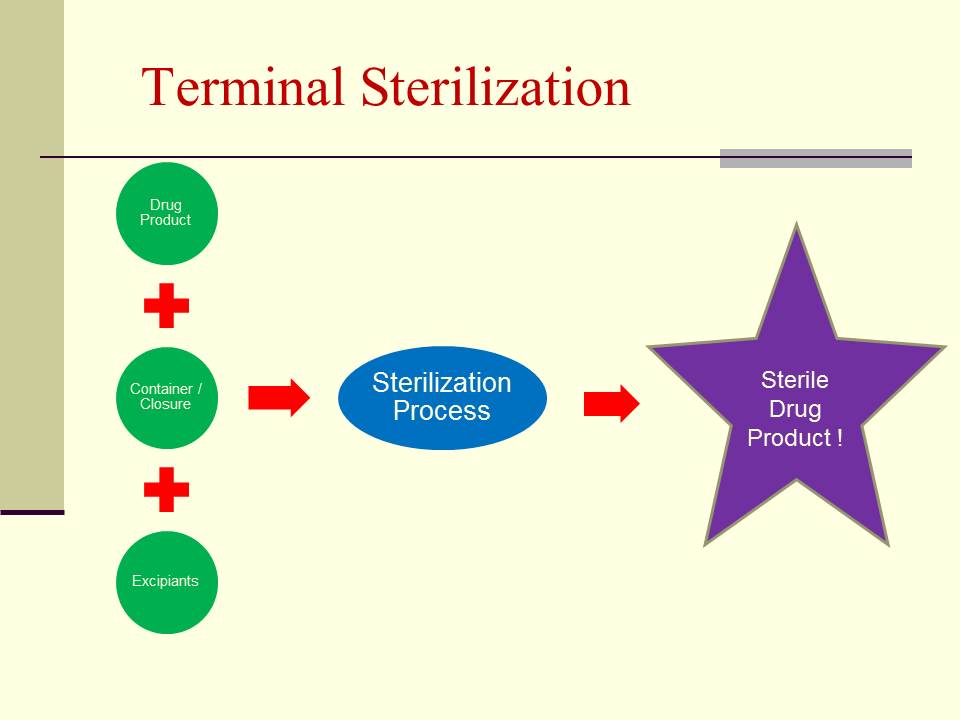
Aseptic Processing vs. Terminal Sterilization
Aseptic Processing: Aseptic processing aims to prevent microbial contamination during the entire manufacturing process. It involves maintaining sterility throughout the production chain, from raw material handling to packaging.
Terminal Sterilization: Terminal sterilization, on the other hand, is a process where the product is sterilized after it has been sealed in its final container. This approach may not be suitable for all products, as some may be heat-sensitive or incompatible with sterilization methods.
Contamination: Sources and Control
Sources of Contamination: Contamination in aseptic processing can originate from personnel, equipment, raw materials, or the environment. It is essential to identify potential sources to implement effective control measures.
Control Measures: Control measures include the use of aseptic cleanrooms, gowning procedures, sterilization of equipment, and air filtration systems to minimize contamination risks.
Microbial Environmental Monitoring
Purpose: Microbial environmental monitoring involves regular testing of the aseptic environment to ensure its integrity and effectiveness in preventing contamination.
Methods: Monitoring methods include surface swabs, settle plates, and air sampling to detect and quantify microbial contamination.
Microbiological Testing of Air and Water
Air Quality: Monitoring the quality of air in cleanrooms is crucial. Testing involves measuring the concentration of airborne particles and microorganisms.
Water Quality: Water used in aseptic processing must also meet stringent quality standards. Testing includes assessing microbial load, endotoxin levels, and chemical contaminants.
Characterization of Aseptic Process
Validation: Validation of the aseptic process is essential to ensure that it consistently produces sterile products. This involves conducting a series of tests and studies to demonstrate its effectiveness.
Risk Assessment: Identifying potential risks within the process and implementing risk mitigation strategies is integral to characterizing aseptic processing.
Media and Incubation Conditions
Media Selection: Appropriate growth media are chosen for microbial testing based on the types of microorganisms expected to be present.
Incubation: Incubation conditions, such as temperature and time, are carefully controlled to encourage the growth of any potential contaminants, making them easier to detect.
Conclusion
Aseptic processing plays a pivotal role in ensuring the safety and efficacy of pharmaceutical and biotechnological products. By preventing microbial contamination throughout the manufacturing process, it allows for the production of sterile products that meet the highest quality standards. Stringent control measures, regular environmental monitoring, and microbiological testing of air and water are essential components of successful aseptic processing.
References
[1] Akers, M. J. (2000). Aseptic processing. Encyclopedia of Biopharmaceutical Statistics, 37-41.
[2] Barbeau, J., Bérubé, D., & Villemur, R. (2002). Detection of airborne methicillin-resistant Staphylococcus aureus in relation to air exchange rates in an experimental hospital ward. Indoor air, 12(1), 19-26.
[3] International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). (2009). ICH Harmonised Tripartite Guideline. Pharmaceutical Quality System Q10. Retrieved from https://database.ich.org/sites/default/files/Q10_Guideline.pdf.
[4] Parenteral Drug Association (PDA). (2017). PDA Technical Report No. 13: Fundamentals of an Environmental Monitoring Program. Retrieved from https://store.pda.org/TableOfContents/620313.pdf.
[5] Rathore, A. S., & Warikoo, V. (2009). Aseptic processing. In Single-use technology in biopharmaceutical manufacture (pp. 247-269). CRC Press.
NOTE
Please note that this article provides an overview of aseptic processing, and the actual practices and regulations may vary depending on specific industries and regions. Always refer to the most up-to-date guidelines and standards in your area of practice.