What is a Primary Standard?
Primary standard is a compound of sufficient purity from which standard solutions of known normalities can be prepared by direct weighing of it and diluting to a defined volume of solution.
Examples
Sodium carbonate Na2CO3
Sodium borate Na2B4O7
Potassium hydrogen iodate KH(IO3)2
Pure metals and their salts like Zn, Mg, Cu, Mn, Ag, AgNO3 , NaCl, KCl, KBr – used in Acid base titrations
K2Cr2O7, KBrO3, KIO3, KI(IO3)2, NaC2O4, As2O3, pure iron – used in redox titrations
Eligibility criteria for a primary standard
A primary standard should satisfy the following conditions

- should be very pure
- should neither be deliquescent (absorbing moisture) nor efflorescent (losing water)
- should have high molecular weight so that weighing errors can be minimised
- should be chemically stable
- shall be readily soluble under given conditions
- it should react stoichiometrically
For more information one can read Benteley and Driver’s Textbook of Qualitative chemical analysis
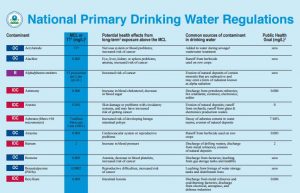
Primary drinking water standards:
Primary standards and treatment techniques protect public health by limiting the levels of contaminants in drinking water.
Microorganisms
Disinfectants
Disinfection Byproducts
Inorganic Chemicals
Organic Chemicals
Radionuclides
Maximum Contaminant Level Goal (MCLG) – The level of a contaminant in drinking water below which there is no known or expected risk to health. MCLGs allow for a margin of safety and are non-enforceable public health goals.
Maximum Contaminant Level (MCL) – The highest level of a contaminant that is allowed in drinking water. MCLs are set as close to MCLGs as feasible using the best available treatment technology and taking cost into consideration. MCLs are enforceable standards.
Maximum Residual Disinfectant Level Goal (MRDLG) – The level of a drinking water disinfectant below which there is no known or expected risk to health. MRDLGs do not reflect the benefits of the use of disinfectants to control microbial contaminants.
Treatment Technique (TT) – A required process intended to reduce the level of a contaminant in drinking water.
Maximum Residual Disinfectant Level (MRDL) – The highest level of a disinfectant allowed in drinking water. There is convincing evidence that addition of a disinfectant is necessary for control of microbial contaminants.
The National Primary Drinking Water Regulations (NPDWR) are here:
Contaminant MCLG1 (mg/L)2 MCL or TT1 (mg/L)2 Potential Health Effects from Long-Term Exposure Above the MCL (unless specified as short-term) Sources of Contaminant in Drinking Water
Cryptosporidium zero TT3
Gastrointestinal illness (such as diarrhea, vomiting, and cramps)
Human and animal fecal waste
Giardia lamblia zero TT3
Gastrointestinal illness (such as diarrhea, vomiting, and cramps)
Human and animal fecal waste
Heterotrophic plate count (HPC) n/a TT3
HPC has no health effects; it is an analytic method used to measure the variety of bacteria that are common in water. The lower the concentration of bacteria in drinking water, the better maintained the water system is.
HPC measures a range of bacteria that are naturally present in the environment
Legionella zero TT3
Legionnaire’s Disease, a type of pneumonia
Found naturally in water; multiplies in heating systems
Total Coliforms (including fecal coliform and E. Coli)
Quick reference guide
zero 5.0%4
Not a health threat in itself; it is used to indicate whether other potentially harmful bacteria may be present5
Coliforms are naturally present in the environment; as well as feces; fecal coliforms and E. coli only come from human and animal fecal waste.
Click here down for primary drinking water standards PDF
primary drinking water standards PDF