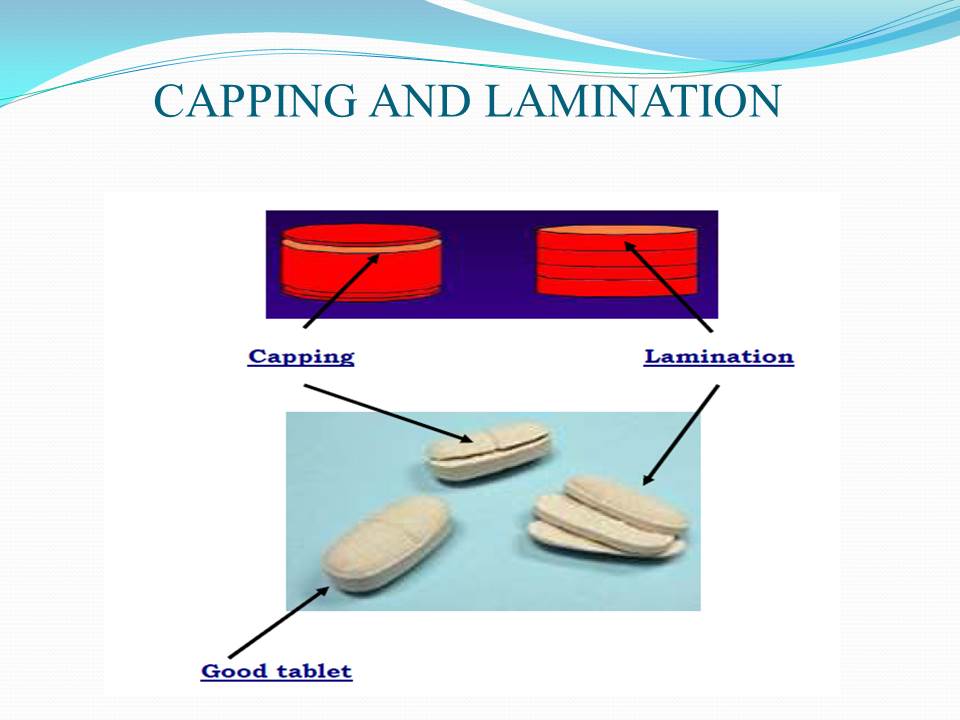
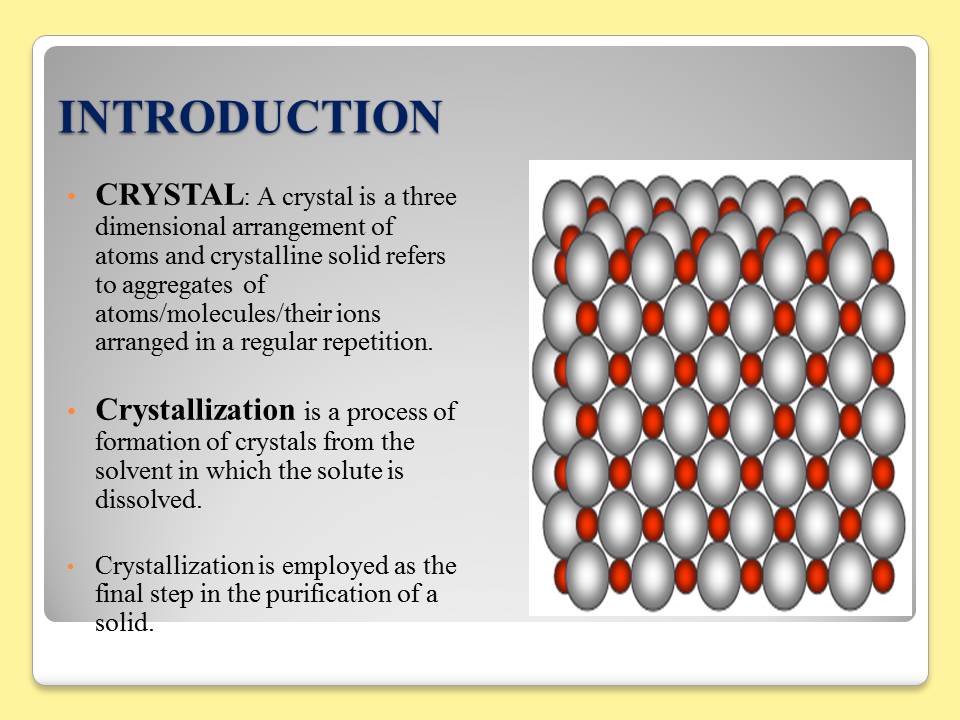
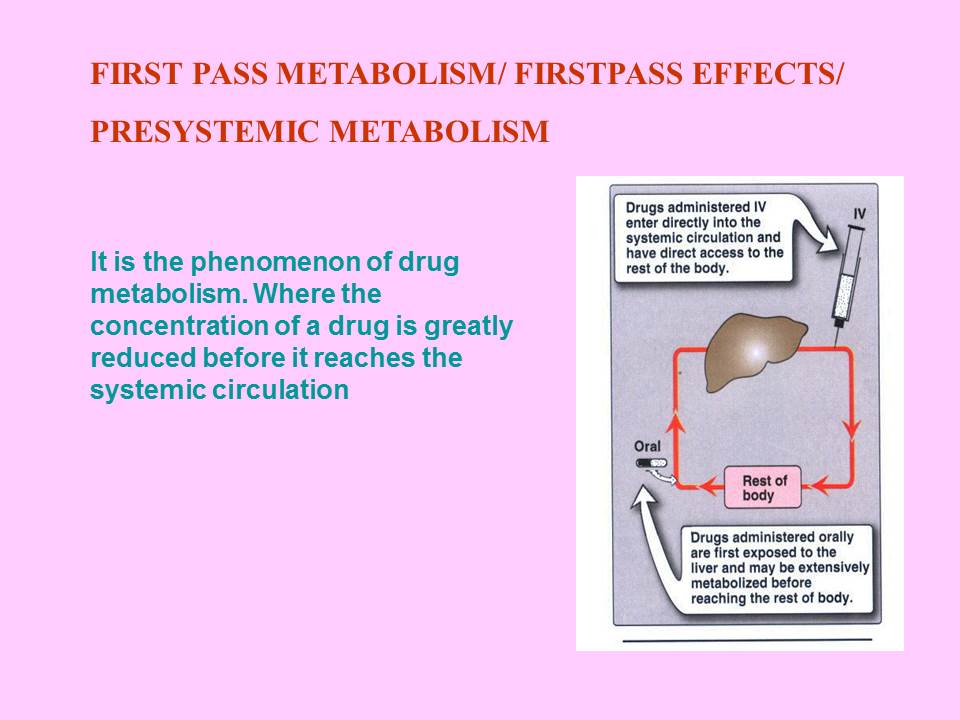
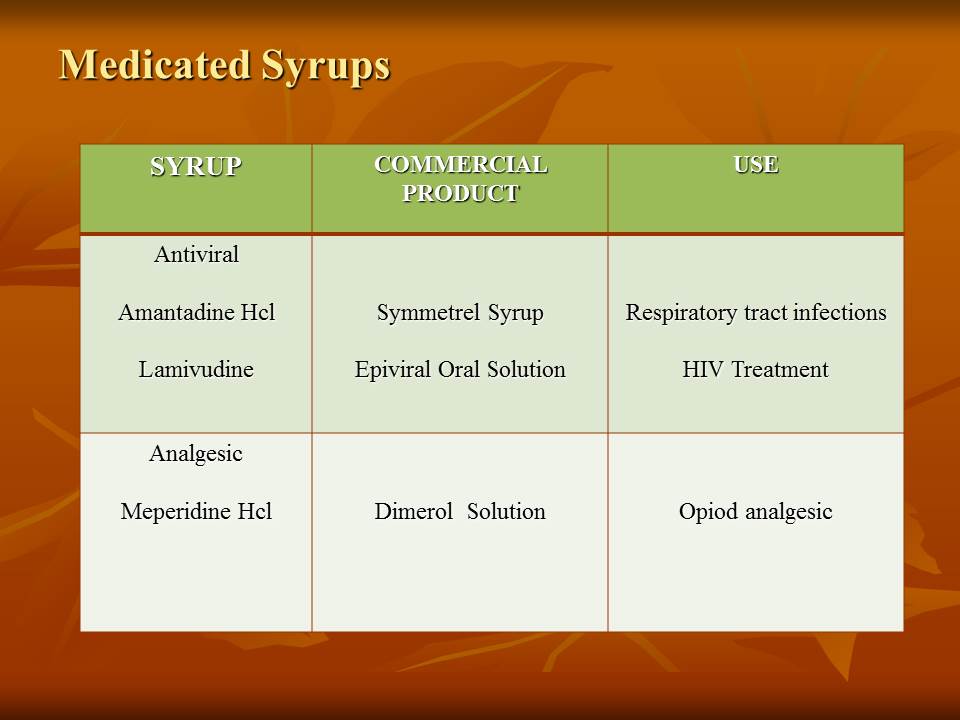
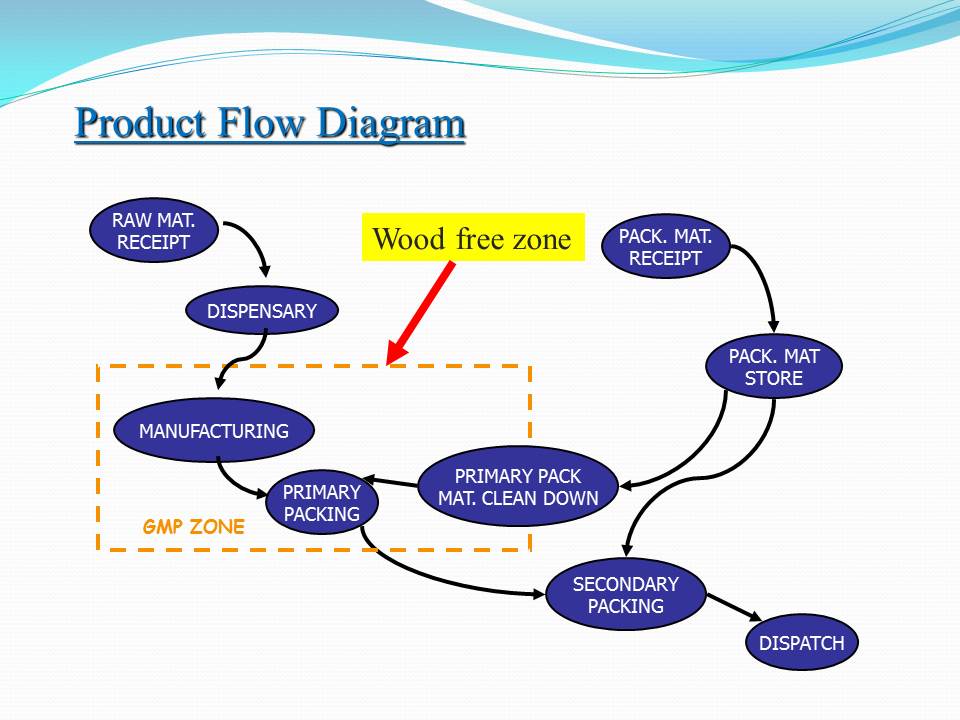
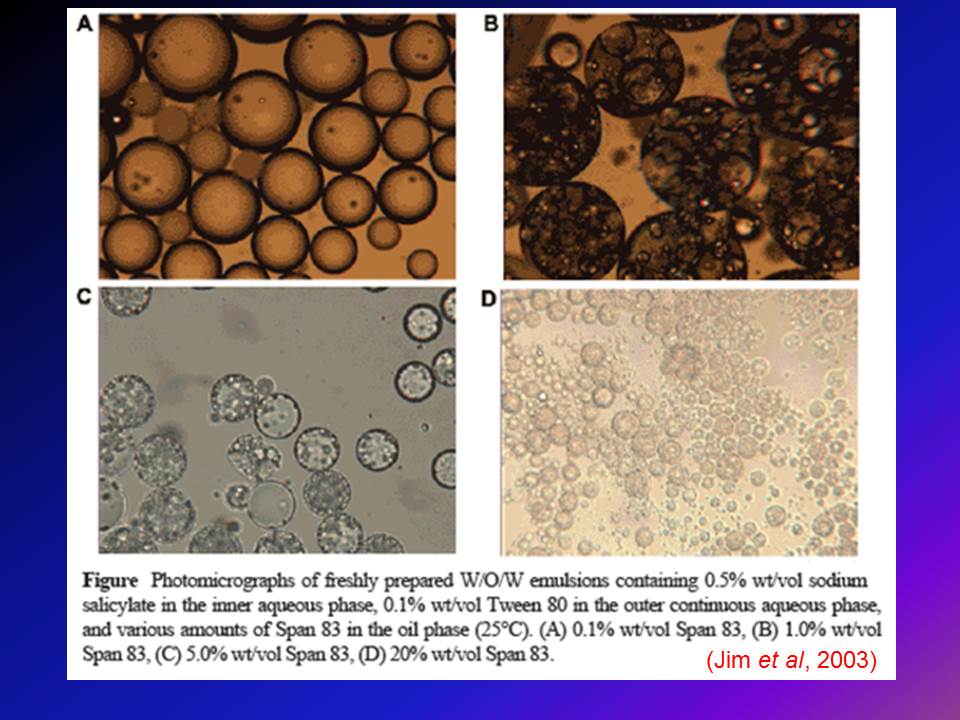
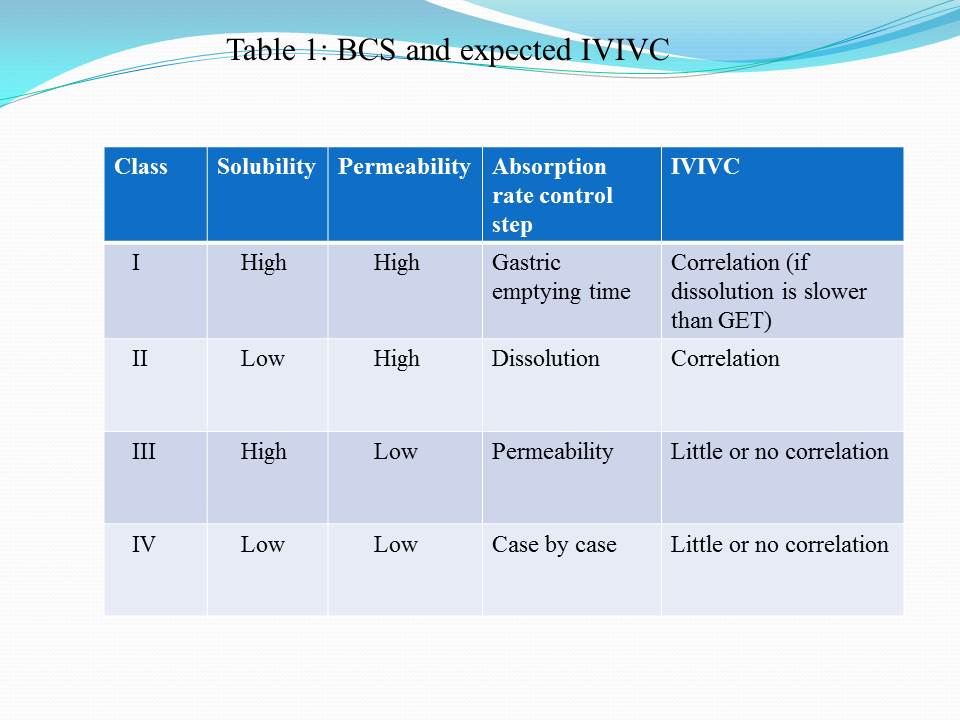
Contents of the powerpoint on Pharmaceutical Plant Design Aspects include:
Introduction
Plant Layout
GMP in Solid dosage forms
GMP in Topical products
GMP in Liquid Orals
GMP in Parenterals
GMP in metered dose inhalers
Conclusion
References
Download the powerpoint by liking us on Facebook
[like-gate][/like-gate]
[button url=”http://pharmawiki.in/?attachment_id=3410″ style=”glass” background=”#1782f9″ color=”#ffffff” size=”4″ center=”yes” icon=”icon: download”]Download PPT here[/button]
You can also download the PDF onPharmaceutical Plant Design Aspects by clicking here
[button url=”http://pharmawiki.in/?attachment_id=3417″ style=”glass” background=”#1782f9″ color=”#ffffff” size=”4″ center=”yes” icon=”icon: download”]Download PDF here[/button]
Stages of pharmaceutical facility planning and design
An independent and objective viewpoint
methods and experience to analyse the process as a whole
modelling and simulation tools
benchmarking data
methods and procedures to formulate a robust basis for the project to proceed.
Site Master Planning
Despite the high-tech image of pharmaceutical facilities, many of today’s manufacturing plants are over 20 years old and have developed in an unstructured manner. Support services will often have been provided individually on a project-by-project basis where, with hindsight, a more holistic approach would have been more cost-effective. Time pressures on new projects may have resulted in new facilities being located in the most convenient position rather than the best location for the overall site development.
Consequently, many companies have identified the need for a more structured planning approach to their future site developments, involving
rationalization of existing site facilities
reduction in operating costs in support services
surveying and census of newly acquired facilities
upgrading for good manufacturing practice (GMP), environmental health and safety (EHS) or to incorporate new technologies
improvements in flows and departmental relationships.
Each pharmaceutical company understands its own business better than any other organization and in this respect the site master plan is best undertaken by the company’s own resource. However, an external services provider can often add value by
bringing an independent and objective approach
providing census techniques, questionnaires and workshops
providing industry benchmarking
offering specialist knowledge in key technology areas
providing resources to enable the study.
Figure 1: Development of the site master plan.
The resulting master plan will provide the cornerstone for future development of the manufacturing site and a framework within which, each future project can fit. The illustration provided in Figure 1 gives an overview of the entire process.
Process Design
Once a new candidate active compound has been identified, a pharmaceutical company sets out to develop the manufacturing process. Almost without exception this results in the generation of a batch process though it is well understood that continuous processes are more efficient and cost only a fraction of the equivalent batch process. Speed-to-market and concerns over validation of the process have meant that a pharmaceutical company’s approach to process development is conservative. From a process engineer’s point of view, it seems that the end result of a huge amount of engineering effort simply produces a scaled-up version of the laboratory plant
Pharmaceutical Facility Design Pharmaceutical Facility Design:
By J. Manfredi PhEn-602 Spring ’09
Architecture & Layout Considerations Architecture & Layout Considerations
Important to understand the manufacturing processes Important to understand the manufacturing processes
and conduct the facility programming. and conduct the facility programming.
Facility Facility layout must be an integrated design that must be an integrated design that
satisfies the following: satisfies the following:
• Process requirements Process requirements
• Personnel flows Personnel flows
• Material flows (product, component and raw material Material flows (product, component and raw material
movements) movements)
• Equipment layout requirements Equipment layout requirements
• Operational access requirements Operational access requirements
• Maintenance access requirements Maintenance access requirements
3
Architecture & Layout Considerations Architecture & Layout Considerations
The layout of the sterile manufacturing facility The layout of the sterile manufacturing facility
must be developed around the needs of the must be developed around the needs of the
facility. facility.
The needs of the facility are defined during The needs of the facility are defined during
the facility programming stage. the facility programming stage.
4
Architecture & Layout Considerations Architecture & Layout Considerations
During the programming phase, the firm must define During the programming phase, the firm must define
their true needs…they must separate the “must their true needs…they must separate the “must
have” objectives from their “wants” objectives. have” objectives from their “wants” objectives.
This is often a very time consuming effort, since each This is often a very time consuming effort, since each
department needs to re department needs to re
-think what is truly think what is truly
mandatory for their operation versus those items mandatory for their operation versus those items
that are desirable, but not essential to successful that are desirable, but not essential to successful
operations. operations.
Formal decision analysis may need to be performed. Formal decision analysis may need to be performed.
5
Architecture & Layout Considerations Architecture & Layout Considerations
Architectural design must consider proper room Architectural design must consider proper room
finishes, environmental and safety considerations, finishes, environmental and safety considerations,
and must ensure that design is compliant with and must ensure that design is compliant with
building codes and fire regulations. building codes and fire regulations.
Structural framework and building exterior finishes Structural framework and building exterior finishes
must take into account the interior room must take into account the interior room
environment (i.e.: Minimize the use of columns and environment (i.e.: Minimize the use of columns and
expansion joints within the cleaner areas of a expansion joints within the cleaner areas of a
manufacturing facility where possible). manufacturing facility where possible).
6
Architecture & Layout Considerations Architecture & Layout Considerations
The architect must build the facility around the The architect must build the facility around the
equipment and systems required for the equipment and systems required for the
process….. process…..
Architect must understand the flow of Architect must understand the flow of
personnel and materials! personnel and materials!
7
Architecture & Layout Considerations Architecture & Layout Considerations
• Area classification and hazards must be reviewed Area classification and hazards must be reviewed
• Are potent compounds involved/handled? Are potent compounds involved/handled?
• Are flammable liquids used in formulations? Are flammable liquids used in formulations?
– Explosion proof design may be required. Explosion proof design may be required.
• Explosion proof panels require sp Explosion proof panels require special construction methods and ecial construction methods and
impact layout issues. impact layout issues.
• Are chemically resistant finishes needed? Are chemically resistant finishes needed?
• Service penetrations and routing of utilities must consider Service penetrations and routing of utilities must consider
interior layout interior layout
– Minimize piping mains above clean areas Minimize piping mains above clean areas
• Route to less clean areas to the extent possible Route to less clean areas to the extent possible
• Location of process viewing panels (visibility) is important Location of process viewing panels (visibility) is important
8
Architecture & Layout Considerations Architecture & Layout Considerations
• The designer must first understand the product The designer must first understand the product
and process requirements. and process requirements.
• Accommodation Schedule is the first step Accommodation Schedule is the first step
Accomodation
Schedule
Conceptual
Layout
Equipment and
Facility Layout
9
Architecture & Layout Considerations Architecture & Layout Considerations
Accommodation Schedule: Accommodation Schedule:
• Defines all areas that can influence unit operations Defines all areas that can influence unit operations
required for manufacturing as well as the relationships required for manufacturing as well as the relationships
and flows between them and flows between them
• Materials and personnel are primary focus Materials and personnel are primary focus
• Can be developed once the process is known Can be developed once the process is known
– All process flow diagrams should be complete All process flow diagrams should be complete
• Also referred to as logic diagrams, or bubble diagrams Also referred to as logic diagrams, or bubble diagrams
10
Accommodation
Schedule
CORRIDOR
Personnel –
Clean
Change
Equipment
Airlock
Preparation
Area
Factory
Change
Aseptic
Change/
Gown
ASEPTIC CORE
AUTOCLAVE
EXTERNAL
AREA
INSPECTION &
SECONDARY
PACKAGING
11
Architecture & Layout Considerations Architecture & Layout Considerations
Conceptual Layout Conceptual Layout
• Derived from Accommodation Schedule and Derived from Accommodation Schedule and
equipment sizing needs equipment sizing needs
• Building blocks of equipment lines are developed Building blocks of equipment lines are developed
• Blocks of rooms are assembled based on necessary Blocks of rooms are assembled based on necessary
adjacencies and process requirement adjacencies and process requirement
12
Architecture & Layout Considerations Architecture & Layout Considerations
Equipment Layout Equipment Layout
• Scaled drawing derived from conceptual layout Scaled drawing derived from conceptual layout
• Defines precise room sizes, structural grids Defines precise room sizes, structural grids
• Access routes Access routes
• Building and fire codes, means of egress are Building and fire codes, means of egress are
established in this phase. Building blocks of established in this phase. Building blocks of
equipment lines are developed equipment lines are developed
• Blocks of rooms are assembled based on necessary Blocks of rooms are assembled based on necessary
adjacencies and process requirements adjacencies and process requirements
• Part of detail design phase of project life cycle Part of detail design phase of project life cycle
13
Architecture & Layout Considerations Architecture & Layout Considerations
After Equipment Layout Drawings are prepared, establish After Equipment Layout Drawings are prepared, establish
Material and Personnel Flows Material and Personnel Flows
• Superimposed on Equipment Layout Drawings Superimposed on Equipment Layout Drawings
• Typically superimposed with directional arrows Typically superimposed with directional arrows
• Primary purpose is to illustrate how to eliminate or minimize Primary purpose is to illustrate how to eliminate or minimize
the potential for contamination of the clean room product the potential for contamination of the clean room product
and personnel. and personnel.
• Layout should prevent cross contamination Layout should prevent cross contamination
• One
-way flow always preferred way flow always preferred
• Provide separate entry and exit ways of possible, particularly Provide separate entry and exit ways of possible, particularly
in changing areas. in changing areas.
• Separate gowning and de Separate gowning and de
-gowning areas always preferred gowning areas always preferred
14
Architecture & Layout Considerations Architecture & Layout Considerations
Material and Personnel Flows Material and Personnel Flows
• One
-way flow is always preferred, as long as all other needs way flow is always preferred, as long as all other needs
can be maintained can be maintained
– Often not possible when retrofitting an existing facility Often not possible when retrofitting an existing facility
• Avoid simultaneous two Avoid simultaneous two
-way flow through a common area way flow through a common area
– Door interlocks and alarms used for prevention Door interlocks and alarms used for prevention
• Gowning areas separated entry from exit Gowning areas separated entry from exit
• Layout should prevent entry of personnel into clean/critical Layout should prevent entry of personnel into clean/critical
areas without first going through gowning room areas without first going through gowning room
• Airlocks should be used between areas of different Airlocks should be used between areas of different
classifications (e.g. between co classifications (e.g. between controlled and critical areas). ntrolled and critical areas).
– Airlocks should have door interlocks to prevent simultaneous two Airlocks should have door interlocks to prevent simultaneous two-way flow way flow
15
Architecture & Layout Considerations Architecture & Layout Considerations
• Personnel flows considered: Personnel flows considered:
– Manufacturing personnel Manufacturing personnel
– Maintenance personnel Maintenance personnel
– Quality control personnel Quality control personnel
16
Architecture & Layout Considerations Architecture & Layout Considerations
• Material flows considered: Material flows considered:
– Raw materials Raw materials
– Finished goods Finished goods
– Waste
– Product (In Product (In
-process, Intermediate & Final) process, Intermediate & Final)
– Equipment Equipment
• Clean and dirty components Clean and dirty components
• Portable equipment Portable equipment
• Product containers Product containers
17
Architecture & Layout Considerations Architecture & Layout Considerations
• Provide sufficient space for operations Provide sufficient space for operations
• Provide sufficient space for movement, equipment access and Provide sufficient space for movement, equipment access and
egress for life safety code requirements egress for life safety code requirements
• Rooms must be sized only after you fully understand what goes in Rooms must be sized only after you fully understand what goes into
the room, and the process that takes place between the four wall the room, and the process that takes place between the four wall
s
• Can’t overlook need for extra space for portable items brought i Can’t overlook need for extra space for portable items brought into
the room, such as carts. the room, such as carts.
• Mechanical and electrical equipment panels also need to be taken Mechanical and electrical equipment panels also need to be taken
into account. into account.
18
Architecture & Layout Considerations Architecture & Layout Considerations
Cost considerations in layout design: Cost considerations in layout design:
• Layout has significant impact on the amount of materials Layout has significant impact on the amount of materials
and therefore facility cost and therefore facility cost
• Minimize perimeter vs. internal area, to reduce costs of Minimize perimeter vs. internal area, to reduce costs of
external load bearing walls and insulation. external load bearing walls and insulation.
• Simple plan shapes are most economical Simple plan shapes are most economical
– Square maximizes internal Square maximizes internal area, minimizes perimeter area, minimizes perimeter
• Minimize building height Minimize building height
• Minimize number and size of clean rooms, particularly Minimize number and size of clean rooms, particularly
Class 100 rooms Class 100 rooms
• Minimize size of clean corridors and staging areas Minimize size of clean corridors and staging areas
19
Architecture & Layout Considerations Architecture & Layout Considerations
Minimize height of building to extent possible. Minimize height of building to extent possible.
Height increases cost due to: Height increases cost due to:
– Increase in amount of perimeter wall for a given total Increase in amount of perimeter wall for a given total
floor area floor area
– Increased load on the structure Increased load on the structure
• Heavier load on columns and footings Heavier load on columns and footings
– Additional hoisting of materials and extra time taken by Additional hoisting of materials and extra time taken by
operators to reach the higher floors operators to reach the higher floors
20
Architecture & Layout Considerations Architecture & Layout Considerations
• Thermal currents Thermal currents
• Unidirectional airflow shading Unidirectional airflow shading
21
GMP’s 21 CFR Part 211 21 CFR Part 211
– Subpart C Subpart C
-Buildings and Facilities Buildings and Facilities
• § 211.42 Design and construction features. § 211.42 Design and construction features.
• (a) Any building or buildings used (a) Any building or buildings used in the manufacture, processin in the manufacture, processing,
packing, or holding of a drug product shall be of packing, or holding of a drug product shall be of suitable size suitable size,
construction and location to fac construction and location to facilitate cleaning, maintenance, a ilitate cleaning, maintenance, and
proper operations. proper operations.
• (b) Any such building shall have (b) Any such building shall have adequate space adequate space for the orderly for the orderly
placement of equipment and materi placement of equipment and materials to prevent mixups between als to prevent mixups between
different components, drug produc different components, drug product containers, closures, labelin t containers, closures, labeling, in
–
process materials, or drug produc process materials, or drug products, and to prevent contaminatio ts, and to prevent contamination.
• The flow of components, drug pr The flow of components, drug product containers, closures, label oduct containers, closures, labeling,
in
-process materials, and drug prod process materials, and drug products through the building or ucts through the building or
buildings shall be buildings shall be designed to prevent contamination designed to prevent contamination.
• (c) Operations shall be performed within specifically defined (c) Operations shall be performed within specifically defined areas of areas of
adequate size adequate size.
22
Example Equipment Layout Example Equipment Layout
23
Architecture & Layout Considerations Architecture & Layout Considerations
• Room criteria sheets help to de Room criteria sheets help to define the requirements upfront (al fine the requirements upfront (also
referred to as Lab Cards) referred to as Lab Cards)
Room Name: Main Compounding Room
General Area: Compounding
Room no. 128
Structural
Hoist
Monorail
Floor pits (scales)
Operational Issues: Three fixed tanks, 100 L, 500 L, 1,000 L
wash down
Special material handling
Purified water Drop (3 use points)
Miscellaneous: Wall bumpers
Roof hatches
Armor plate on doors
Shelving
Storage cabinet
24
Architecture & Layout Considerations Architecture & Layout Considerations
Gowning rooms play a critical role in the facility layout. Gowning rooms play a critical role in the facility layout.
Cleanroom Cleanroom clothing: clothing:
• Designed to limit the rate of particle generation from the Designed to limit the rate of particle generation from the
person
• Designed to limit the rate of particle generation from the clean Designed to limit the rate of particle generation from the clean
room garment. room garment.
• In cleanrooms where contamination is not as important (e.g. In cleanrooms where contamination is not as important (e.g.
pharmaceutical areas and Class 100,000 areas), smock, cap and pharmaceutical areas and Class 100,000 areas), smock, cap and
shoe covers may be appropriate. shoe covers may be appropriate.
25
Architecture & Layout Considerations Architecture & Layout Considerations
Changing rooms: Changing rooms:
Two grades (levels) of changing rooms Two grades (levels) of changing rooms
• Low (standard) Low (standard)
– From normal clothing (street clothes) to factory From normal clothing (street clothes) to factory
(clean) clothing (clean) clothing
• High (standard) High (standard)
– From clean clothing to full coverage suit From clean clothing to full coverage suit
26
Architecture & Layout Considerations Architecture & Layout Considerations
Cleanroom clothing requirements are found in: Cleanroom clothing requirements are found in:
• IEST Recommended Practice RP IEST Recommended Practice RP
-CC
-003.2
• EU Guidelines EU Guidelines
27
Architecture & Layout Considerations Architecture & Layout Considerations
Cleanroom clothing: Cleanroom clothing:
• In cleanrooms where contamination is In cleanrooms where contamination is
critical, (e.g. Class 10,000 and Class 100 critical, (e.g. Class 10,000 and Class 100
areas), a full coverage coverall, hood, boots, areas), a full coverage coverall, hood, boots,
mask, gloves and goggles are worn. mask, gloves and goggles are worn.
28
Architecture & Layout Considerations Architecture & Layout Considerations
Gray zones: Service space or maintenance Gray zones: Service space or maintenance
space typically adjacent to the production space typically adjacent to the production
room
• Contains the majority of piping, valves, electrical Contains the majority of piping, valves, electrical
conduit and other utilities that support the conduit and other utilities that support the
manufacturing area. manufacturing area.
• Maintenance personnel have separate access to Maintenance personnel have separate access to
these areas, allowing less stringent gowning these areas, allowing less stringent gowning
requirements, and allows for maintenance requirements, and allows for maintenance
without shutting down or disrupting the without shutting down or disrupting the
manufacturing operation manufacturing operation
29
Example Layout Example Layout
Material
s Airlock
Change room/
Gowning Area
OVEN
PREPARATION
AREA
ASEPTIC CORRIDOR
ASEPTIC
FILLING
ROOM
AREA
30
Material & Personnel Flow Example Material & Personnel Flow Example
Materials
Airlock
Change room/
Exit
OVEN
PERSONNEL ENTRY
ASEPTIC CORRIDOR
ASEPTIC
FILLING
ROOM
AREA
GOWNING
AREA – IN
MATERIAL ENTRY
31
Desirable Desirable
Layout
Aseptic Core
Receiving Dock
Receiving Dept.
Incoming Materials
Wash and
Prep
Controlled Pharm Corridor
Final
Aseptic
Processing
Secondary Packaging
Shipping Dept.
Loading Dock
32
Less Desirable Layout Less Desirable Layout
Loading Dock
Shipping and
Receiving
Secondary
Packaging
Aseptic Core
Wash and
Prep
Final Aseptic
Processing
33
Materials of Construction & Materials of Construction &
Surface Finishes Surface Finishes
34
Materials of Construction & Materials of Construction &
Surface Finishes Surface Finishes
•
§ 211.42 Design and construction features. 211.42 Design and construction features.
• There shall be separate or defined areas for the There shall be separate or defined areas for the
firm’s operations to prevent contamination or firm’s operations to prevent contamination or
mixups as follows: as follows:
• (10) Aseptic processing, which includes as (10) Aseptic processing, which includes as
appropriate: appropriate:
• (I) Floors, walls, and ceilings of smooth, hard Floors, walls, and ceilings of smooth, hard
surfaces that are easily cleanable; surfaces that are easily cleanable;
35
Materials of Construction & Materials of Construction &
Surface Finishes Surface Finishes
• There is no such thing as FDA endorsed materials There is no such thing as FDA endorsed materials
• Surface finishes should be smooth, non Surface finishes should be smooth, non
-shedding, non shedding, non
–
porous, and resistant to sustaining microbial growth porous, and resistant to sustaining microbial growth
• Finishes must withstand repeated cleaning and Finishes must withstand repeated cleaning and
sanitization* without evidence of rust, or peeling paint. sanitization* without evidence of rust, or peeling paint.
*Cleaning and sanitization agents include *Cleaning and sanitization agents include
detergents and disinfectants, as well as hot WFI. detergents and disinfectants, as well as hot WFI.
• Stainless steel often used throughout the facility Stainless steel often used throughout the facility
because of its appearance, durability, smoothness, and because of its appearance, durability, smoothness, and
resistance to rust, peeling and shedding resistance to rust, peeling and shedding
36
Materials of Construction & Surface
Finishes
• Ledges, joints, and corners Ledges, joints, and corners difficult to reach should difficult to reach should be minimized be minimized
• Door hardware should be minimized Door hardware should be minimized
– Use proximity sensors wherever possible Use proximity sensors wherever possible
• A cleanroom cleanroom should be built airtight, where possible should be built airtight, where possible
• Internal surfaces smooth and suitable for cleaning Internal surfaces smooth and suitable for cleaning
• Surfaces must be resistant to impact Surfaces must be resistant to impact
• Joints should be free of openings that could harbor Joints should be free of openings that could harbor
dirt or microbes dirt or microbes
• Crack and crevice Crack and crevice
-free construction free construction
37
Materials of Construction & Surface
Finishes
• Concealed, sealed sprinklers should be used to avoid Concealed, sealed sprinklers should be used to avoid
communication between communication between cleanroom cleanroom and interstitial and interstitial
space
• Electrical outlets should be covered/sealed suitable Electrical outlets should be covered/sealed suitable
for washdown washdown service service
• Predetermined routes for removing/installing tanks Predetermined routes for removing/installing tanks
and other stationary equipment and other stationary equipment
– Removable wall panels often used to avoid tear Removable wall panels often used to avoid tear-out later. out later.
• Bumper guards on doors and corridors that are Bumper guards on doors and corridors that are
subjected to heavy equipment travel subjected to heavy equipment travel
38
Materials of Construction & Surface
Finishes
• Platforms typically stainless steel, including Platforms typically stainless steel, including
decking, stairs and support structure decking, stairs and support structure
• Stainless steel screens on HEPA filters Stainless steel screens on HEPA filters
• Stainless steel benches for gowning areas Stainless steel benches for gowning areas
• Recessed fire extinguishers with stainless steel Recessed fire extinguishers with stainless steel
frame
• All access panels stainless steel All access panels stainless steel
39
Materials of Construction & Surface Materials of Construction & Surface
Finishes Finishes
40
Materials of Construction & Surface
Finishes
Flooring: Flooring: Consider aesthetics, durability and cleanability Consider aesthetics, durability and cleanability
• Epoxy terrazzo: Epoxy terrazzo:
– hard, durable long hard, durable long-lasting surface with excellent chemical resistance lasting surface with excellent chemical resistance
– Very expensive to install Very expensive to install
• Epoxy Resin Systems: (e.g. Epoxy Resin Systems: (e.g. Stonhard Stonhard)
– Very popular in the Pharmaceutical industry Very popular in the Pharmaceutical industry
– Easier to install than Terrazzo Easier to install than Terrazzo
– Moderate durability and chemical resistance Moderate durability and chemical resistance
– Moderate price Moderate price
• Welded Vinyl/PVC Sheet (e.g. Welded Vinyl/PVC Sheet (e.g. Mipolam Mipolam)
– Durability an issue in high Durability an issue in high
-traffic areas traffic areas
– Often used with identical wall system for matching Often used with identical wall system for matching
41
Materials of Construction & Surface
Finishes
Mipolam Mipolam
42
Materials of Construction & Surface Finishes
Typical seamless epoxy resin flooring system Typical seamless epoxy resin flooring system
1.Polyurethane
Coating
(Gloss or Matte)
2.Epoxy Clear Coat
3.Broadcast
4.Epoxy Coat
5.Heavy Duty Mortar
6.CornerCrete Slurry
Primer
7.Substrate
43
• Epoxy terrazzo: Epoxy terrazzo:
– hard, durable long hard, durable longlasting
surface with lasting surface with
excellent chemical excellent chemical
resistance resistance
– Very expensive to Very expensive to
install install
Flooring Flooring
44
Radius cove base typically Radius cove base
used
Materials of Construction & Surface
Finishes
Radius cove base in modular
construction
45
Materials of Construction & Surface
Finishes
Flooring/drains Flooring/drains
• Drains should be avoided in Class 100 Drains should be avoided in Class 100
through Class 10,000 rooms. through Class 10,000 rooms.
• Acceptable in Class 100,000 rooms Acceptable in Class 100,000 rooms
46
Materials of Construction & Surface
Finishes
Ceiling Systems Ceiling Systems
• Same issues are important: aesthetics, durability and cleanabili Same issues are important: aesthetics, durability and cleanability
• Gypsum Wall Gypsum Wall-board (GWB) with finish coat of epoxy paint board (GWB) with finish coat of epoxy paint
– Pro’s:
• Flexible, easy to use Flexible, easy to use
• Most common in the industry Most common in the industry
– Con’s:
• Restricts future above Restricts future above
-ceiling access ceiling access
• Requires careful coordination with other trades during Requires careful coordination with other trades during
construction. (e.g. piping penetr construction. (e.g. piping penetrations, HVAC diffusers, light ations, HVAC diffusers, light
fixtures, filter housings). fixtures, filter housings).
47
Materials of Construction & Surface Finishes
Ceiling Systems Ceiling Systems
• Lay
-in grid ceilings ( in grid ceilings (http:// http://www.cleanroomeng.com/products_ceiling.cfm www.cleanroomeng.com/products_ceiling.cfm)
• Use gasketed clean Use gasketed clean
-room tiles room tiles
• Seams and joints still a potenti Seams and joints still a potential breeding ground for bacteria al breeding ground for bacteria
48
Materials of Construction & Surface Finishes
Light fixtures Light fixtures
• Lay
-in grid ceilings in grid ceilings
– sealed units with stainless trim sealed units with stainless trim
• Teardrop Teardrop
49
For Class 100 through For Class 100 through
Class 10,000 (Grade A and Class 10,000 (Grade A and
Grade B areas), radius Grade B areas), radius
coves are often used in the coves are often used in the
ceiling to wall junction as well ceiling to wall junction as well
Materials of Construction & Surface Finishes
Radius cove where ceiling
meets wall. (modular
construction shown).
50
Materials of Construction & Surface Finishes
Wall systems Wall systems
• Consider aesthetics, durability and cleanability Consider aesthetics, durability and cleanability
• Gypsum Wall Gypsum Wall
-board (GWB) with epoxy paint board (GWB) with epoxy paint
finish
• Concrete Block/concrete block with plaster Concrete Block/concrete block with plaster
• Epoxy Resin walls Epoxy Resin walls
• Welded sheet PVC (e.g. Welded sheet PVC (e.g. Mipolam Mipolam)
– Typically used in higher class areas (e.g. Class 100) Typically used in higher class areas (e.g. Class 100)
51
Materials of Construction & Surface Finishes
Wall systems Wall systems
(Vision Panels) (Vision Panels)
• Flush with the wall to Flush with the wall to
eliminate ledges eliminate ledges
• Often Stainless frame, Often Stainless frame,
double pane double pane
• Free of gaps along the Free of gaps along the
perimeter perimeter
52
Materials of Construction & Surface Finishes
Cleanroom Doors
Full glass or half glass architectural aluminum doors are available with a
variety of hardware options, including pivot hinges, surface mounted closers,
concealed closers, panic hardware, and locksets.
53
Materials of Construction & Surface Finishes
Steel Doors
Glazed or flush steel doors integrate into the aluminum door frames utilizing butt
hinges at the jamb connection.
54
Materials of Construction & Surface Finishes
Electric Sliding
Sliding doors easily integrate into wall systems and are available in any size with
a variety of hardware available including push button entries and motion sensors.
55
Materials of Construction & Surface Finishes
High
-Speed Roll Speed Roll-Up Doors Up Doors
Roll Up Doors offer advantages over swinging doors, sliding doors, and strip
curtains. Doors can be created up to 18′ x 18′. Suitable for Class 100,000 to Class
10,000 applications. The door opens and closes quickly reducing the time the
cleanroom interior is exposed.
56
Materials of Construction & Surface Finishes
Glazing Glazing
Windows and doors can be glazed with a variety of options as specified by the
project requirements. One can choose from tempered glass, tinted glass, Lexan,
acrylic, static dissipative, film-covered glass or solid panels
57
Materials of Construction & Surface Finishes
AIR SHOWERS
Chambers located between the clean room and an outside environment that
remove particulate contamination from clean room garments as personnel pass
through. The chambers may include HEPA filters, interlocking doors, a recirculating
air system, and air nozzles in various patterns through which filtered
air is blown onto the personnel in the shower. The high-velocity air is moved over
the worker, removing particulate contamination from the worker’s garments.
58
Materials of Construction & Surface Finishes
Modular Modular Cleanroom Cleanroom Facilities Facilities
Self-contained facilities built off contained facilities built off-site under controlled site under controlled
conditions, then delivered and integrated into the final conditions, then delivered and integrated into the final
point of use location with the minimal amount of point of use location with the minimal amount of
reassembly. reassembly.
• Very popular alternative. Very popular alternative.
Three ISPE Facility of the Year Awards were given to Three ISPE Facility of the Year Awards were given to
firms that utilized the modular concept. firms that utilized the modular concept.
– Novo
-Nordisk, Denmark Nordisk, Denmark
– Baxter Biopharma Biopharma Solutions, Bloomington, Indiana Solutions, Bloomington, Indiana
– GSK
59
Modular Clean Modular Clean
-room Facilities room Facilities
• Pre
-engineered, pre engineered, pre
-fabricated walls and top decks that fabricated walls and top decks that
can support weight of air handling equipment, ceilings can support weight of air handling equipment, ceilings
and filters. and filters.
• Can assemble relatively quickly in the field. Can assemble relatively quickly in the field.
• One source for virtually everything for the One source for virtually everything for the cleanroom cleanroom,
airhandling airhandling, HEPA filters, wall panels, lighting, , HEPA filters, wall panels, lighting,
windows, doors, instrumentation and controls, all built windows, doors, instrumentation and controls, all built
into the package. into the package.
• Wall and ceiling systems typically of metal panels with Wall and ceiling systems typically of metal panels with
special coatings that resist cleaning agents and give special coatings that resist cleaning agents and give
aesthetically pleasing look aesthetically pleasing look.
• Can opt for PVC finished panels or stainless steel finish Can opt for PVC finished panels or stainless steel finish
panels also. panels also.
Materials of Construction & Surface Finishes
60
Materials of Construction & Surface Finishes
Modular Clean Modular Clean
-room Facilities room Facilities
– Sample Manufacturer Sample Manufacturer
PharmaWall System from PortaFab.
“Designed specifically for pharmaceutical
and biotechnology facilities, the
PharmaWall System utilizes a patent
pending “Z” clip design that integrates
with our 1/2″ (12.7 mm) thick panels to be
hung off a metal stud framework or an
existing wall. The non-progressive system
allows for the easy removal of panels.
By connecting to a metal stud framework
you no longer have to be limited with the
depth of your utility chases. All piping,
mechanical and electrical processes can be
easily integrated within 3-5/8″, 6″, 12″ or
18″ cavities without having to create a
“double” wall out of two free-standing
partition walls.”
61
Materials of Construction & Surface Finishes
Modular Clean -room Facilities – Sample Manufacturer
PharmaWall System from PortaFab. Process & Utility Integration “Piping penetrations and enclosures can be easily created by utilizing the PharmaWall system design. The radius cove details and cleanable surfaces meet the requirements for a clean space and allow for future piping expansion capability. Process service panels are easily integrated into the PharmaWall system in a recessed manner that minimizes ledges and joints. The panels can be designed to allow maintenance access and provide for future piping expansion. The PharmaSystem’s modular design also allows cleanroom designers and contractors the ability to make field modifications during the installation process. This flexibility provides for a number of potential design cost savings.”
62
Materials of Construction & Surface Finishes
Modular Clean Room Facilities: Modular Clean Room Facilities:
• Reasons why modular construction may be used: Reasons why modular construction may be used:
– Compressed construction time Compressed construction time
– Flexibility for future changes Flexibility for future changes
– Other reasons: Other reasons:
• Overcome local skill shortages Overcome local skill shortages
• Reduced weight Reduced weight
• Reduce number of contractors used during construction Reduce number of contractors used during construction
– Rely on the modular firm? Rely on the modular firm?
• Pre
-qualification testing and customer inspection of the qualification testing and customer inspection of the
facilities are completed before shipment to assure facilities are completed before shipment to assure
compliance. compliance.
• Increased safety (Transferred to vendor?) Increased safety (Transferred to vendor?)
63
Materials of Construction & Surface Finishes
Modular Clean Room Facilities Modular Clean Room Facilities
Impediments to use of modular construction: Impediments to use of modular construction:
• Generally more expensive than the traditional “stick Generally more expensive than the traditional “stickbuilt”
clean room built” clean room
• Increased engineering costs Increased engineering costs
• Early design freeze which may reduce flexibility of scope Early design freeze which may reduce flexibility of scope
• Complicated interface (coordination) issues Complicated interface (coordination) issues
• Absence of a robust economic advantage Absence of a robust economic advantage
• Must complete flooring, sprinklers, and other utilities in Must complete flooring, sprinklers, and other utilities in
the field (i.e.: water, electric, specialty gases, etc) the field (i.e.: water, electric, specialty gases, etc)
64
Room Pressurization Room Pressurization
• Both US and EU requires that rooms of higher grade must be Both US and EU requires that rooms of higher grade must be
at higher pressure levels. at higher pressure levels.
• Typically 0.05” water column Typically 0.05” water column difference between classes. difference between classes.
• Ensures air flows from cleaner ar Ensures air flows from cleaner areas to dirtier areas. Class eas to dirtier areas. Class
100 filling rooms always have the highest pressure. 100 filling rooms always have the highest pressure.
• Class 100 fill rooms will sometimes have regions that are class Class 100 fill rooms will sometimes have regions that are class
10,000 (there is no requirement 10,000 (there is no requirement to have the entire room at to have the entire room at
class 100) however the room is still considered a class 100 class 100) however the room is still considered a class 100
room in terms of pressure levels. room in terms of pressure levels.
External Areas
Streets, Offices, Restaurant
Raw
Materials People
Remove
Outers
Sterilize
Change Change Remove Outers
Container/Closures
Compounding
Critical Processing Area
E.g.: Point of Fill
Transition Zone
Brings people, materials, etc. from
external areas to the manufacturing
areas in a “controlled” manner
Clean Area
Provides a protective
envelope to minimize the
challenge to the Critical Areas
66
FILLING
ROOM CLASS
100
REGION
CLASS 10,000
BACKGROUND
ASEPTIC CORRIDOR
CLASS 10,000
CLASS 100,000
CORRIDOR
CLASS 100,000
CORRIDOR
CLASS 100,000
PREPARATION AREA
PHARMACEUTICAL
AREA
PHARMACEUTICAL AREA
LOCKER ROOM – ENTRY AREA
Classification Levels within an Aseptic Facility
Nested Manufacturing Zones (Five Zones)
67
Clean Room Pressurization – Example
Sample Sterile
Manufacturing Suite Airflow
Direction
Building
Corridor
0.00”
Prep
Room
Class
100,000
0.05”
Aseptic
Processing
Class 10,000
0.10”
Filling Room
Class 100
Highest Pressure
0.15”
68
Another
example
of
cascading
room
pressures
Example of an actual facility layout
Following information is collected here and is for only educative and informative purpose.
Pharmaceutical Facility Design Pharmaceutical Facility Design
J. Manfredi J. Manfredi
2
Architecture & Layout Considerations Architecture & Layout Considerations
Important to understand the manufacturing processes Important to understand the manufacturing processes
and conduct the facility programming. and conduct the facility programming.
Facility Facility layout must be an integrated design that must be an integrated design that
satisfies the following: satisfies the following:
• Process requirements Process requirements
• Personnel flows Personnel flows
• Material flows (product, component and raw material Material flows (product, component and raw material
movements) movements)
• Equipment layout requirements Equipment layout requirements
• Operational access requirements Operational access requirements
• Maintenance access requirements Maintenance access requirements
3
Architecture & Layout Considerations Architecture & Layout Considerations
The layout of the sterile manufacturing facility The layout of the sterile manufacturing facility
must be developed around the needs of the must be developed around the needs of the
facility. facility.
The needs of the facility are defined during The needs of the facility are defined during
the facility programming stage. the facility programming stage.
4
Architecture & Layout Considerations Architecture & Layout Considerations
During the programming phase, the firm must define During the programming phase, the firm must define
their true needs…they must separate the “must their true needs…they must separate the “must
have” objectives from their “wants” objectives. have” objectives from their “wants” objectives.
This is often a very time consuming effort, since each This is often a very time consuming effort, since each
department needs to re department needs to re
-think what is truly think what is truly
mandatory for their operation versus those items mandatory for their operation versus those items
that are desirable, but not essential to successful that are desirable, but not essential to successful
operations. operations.
Formal decision analysis may need to be performed. Formal decision analysis may need to be performed.
5
Architecture & Layout Considerations Architecture & Layout Considerations
Architectural design must consider proper room Architectural design must consider proper room
finishes, environmental and safety considerations, finishes, environmental and safety considerations,
and must ensure that design is compliant with and must ensure that design is compliant with
building codes and fire regulations. building codes and fire regulations.
Structural framework and building exterior finishes Structural framework and building exterior finishes
must take into account the interior room must take into account the interior room
environment (i.e.: Minimize the use of columns and environment (i.e.: Minimize the use of columns and
expansion joints within the cleaner areas of a expansion joints within the cleaner areas of a
manufacturing facility where possible). manufacturing facility where possible).
6
Architecture & Layout Considerations Architecture & Layout Considerations
The architect must build the facility around the The architect must build the facility around the
equipment and systems required for the equipment and systems required for the
process….. process…..
Architect must understand the flow of Architect must understand the flow of
personnel and materials! personnel and materials!
7
Architecture & Layout Considerations Architecture & Layout Considerations
• Area classification and hazards must be reviewed Area classification and hazards must be reviewed
• Are potent compounds involved/handled? Are potent compounds involved/handled?
• Are flammable liquids used in formulations? Are flammable liquids used in formulations?
– Explosion proof design may be required. Explosion proof design may be required.
• Explosion proof panels require sp Explosion proof panels require special construction methods and ecial construction methods and
impact layout issues. impact layout issues.
• Are chemically resistant finishes needed? Are chemically resistant finishes needed?
• Service penetrations and routing of utilities must consider Service penetrations and routing of utilities must consider
interior layout interior layout
– Minimize piping mains above clean areas Minimize piping mains above clean areas
• Route to less clean areas to the extent possible Route to less clean areas to the extent possible
• Location of process viewing panels (visibility) is important Location of process viewing panels (visibility) is important
8
Architecture & Layout Considerations Architecture & Layout Considerations
• The designer must first understand the product The designer must first understand the product
and process requirements. and process requirements.
• Accommodation Schedule is the first step Accommodation Schedule is the first step
Accomodation
Schedule
Conceptual
Layout
Equipment and
Facility Layout
9
Architecture & Layout Considerations Architecture & Layout Considerations
Accommodation Schedule: Accommodation Schedule:
• Defines all areas that can influence unit operations Defines all areas that can influence unit operations
required for manufacturing as well as the relationships required for manufacturing as well as the relationships
and flows between them and flows between them
• Materials and personnel are primary focus Materials and personnel are primary focus
• Can be developed once the process is known Can be developed once the process is known
– All process flow diagrams should be complete All process flow diagrams should be complete
• Also referred to as logic diagrams, or bubble diagrams Also referred to as logic diagrams, or bubble diagrams
10
Accommodation
Schedule
CORRIDOR
Personnel –
Clean
Change
Equipment
Airlock
Preparation
Area
Factory
Change
Aseptic
Change/
Gown
ASEPTIC CORE
AUTOCLAVE
EXTERNAL
AREA
INSPECTION &
SECONDARY
PACKAGING
11
Architecture & Layout Considerations Architecture & Layout Considerations
Conceptual Layout Conceptual Layout
• Derived from Accommodation Schedule and Derived from Accommodation Schedule and
equipment sizing needs equipment sizing needs
• Building blocks of equipment lines are developed Building blocks of equipment lines are developed
• Blocks of rooms are assembled based on necessary Blocks of rooms are assembled based on necessary
adjacencies and process requirement adjacencies and process requirement
12
Architecture & Layout Considerations Architecture & Layout Considerations
Equipment Layout Equipment Layout
• Scaled drawing derived from conceptual layout Scaled drawing derived from conceptual layout
• Defines precise room sizes, structural grids Defines precise room sizes, structural grids
• Access routes Access routes
• Building and fire codes, means of egress are Building and fire codes, means of egress are
established in this phase. Building blocks of established in this phase. Building blocks of
equipment lines are developed equipment lines are developed
• Blocks of rooms are assembled based on necessary Blocks of rooms are assembled based on necessary
adjacencies and process requirements adjacencies and process requirements
• Part of detail design phase of project life cycle Part of detail design phase of project life cycle
13
Architecture & Layout Considerations Architecture & Layout Considerations
After Equipment Layout Drawings are prepared, establish After Equipment Layout Drawings are prepared, establish
Material and Personnel Flows Material and Personnel Flows
• Superimposed on Equipment Layout Drawings Superimposed on Equipment Layout Drawings
• Typically superimposed with directional arrows Typically superimposed with directional arrows
• Primary purpose is to illustrate how to eliminate or minimize Primary purpose is to illustrate how to eliminate or minimize
the potential for contamination of the clean room product the potential for contamination of the clean room product
and personnel. and personnel.
• Layout should prevent cross contamination Layout should prevent cross contamination
• One
-way flow always preferred way flow always preferred
• Provide separate entry and exit ways of possible, particularly Provide separate entry and exit ways of possible, particularly
in changing areas. in changing areas.
• Separate gowning and de Separate gowning and de
-gowning areas always preferred gowning areas always preferred
14
Architecture & Layout Considerations Architecture & Layout Considerations
Material and Personnel Flows Material and Personnel Flows
• One
-way flow is always preferred, as long as all other needs way flow is always preferred, as long as all other needs
can be maintained can be maintained
– Often not possible when retrofitting an existing facility Often not possible when retrofitting an existing facility
• Avoid simultaneous two Avoid simultaneous two
-way flow through a common area way flow through a common area
– Door interlocks and alarms used for prevention Door interlocks and alarms used for prevention
• Gowning areas separated entry from exit Gowning areas separated entry from exit
• Layout should prevent entry of personnel into clean/critical Layout should prevent entry of personnel into clean/critical
areas without first going through gowning room areas without first going through gowning room
• Airlocks should be used between areas of different Airlocks should be used between areas of different
classifications (e.g. between co classifications (e.g. between controlled and critical areas). ntrolled and critical areas).
– Airlocks should have door interlocks to prevent simultaneous two Airlocks should have door interlocks to prevent simultaneous two-way flow way flow
15
Architecture & Layout Considerations Architecture & Layout Considerations
• Personnel flows considered: Personnel flows considered:
– Manufacturing personnel Manufacturing personnel
– Maintenance personnel Maintenance personnel
– Quality control personnel Quality control personnel
16
Architecture & Layout Considerations Architecture & Layout Considerations
• Material flows considered: Material flows considered:
– Raw materials Raw materials
– Finished goods Finished goods
– Waste
– Product (In Product (In
-process, Intermediate & Final) process, Intermediate & Final)
– Equipment Equipment
• Clean and dirty components Clean and dirty components
• Portable equipment Portable equipment
• Product containers Product containers
17
Architecture & Layout Considerations Architecture & Layout Considerations
• Provide sufficient space for operations Provide sufficient space for operations
• Provide sufficient space for movement, equipment access and Provide sufficient space for movement, equipment access and
egress for life safety code requirements egress for life safety code requirements
• Rooms must be sized only after you fully understand what goes in Rooms must be sized only after you fully understand what goes into
the room, and the process that takes place between the four wall the room, and the process that takes place between the four wall
s
• Can’t overlook need for extra space for portable items brought i Can’t overlook need for extra space for portable items brought into
the room, such as carts. the room, such as carts.
• Mechanical and electrical equipment panels also need to be taken Mechanical and electrical equipment panels also need to be taken
into account. into account.
18
Architecture & Layout Considerations Architecture & Layout Considerations
Cost considerations in layout design: Cost considerations in layout design:
• Layout has significant impact on the amount of materials Layout has significant impact on the amount of materials
and therefore facility cost and therefore facility cost
• Minimize perimeter vs. internal area, to reduce costs of Minimize perimeter vs. internal area, to reduce costs of
external load bearing walls and insulation. external load bearing walls and insulation.
• Simple plan shapes are most economical Simple plan shapes are most economical
– Square maximizes internal Square maximizes internal area, minimizes perimeter area, minimizes perimeter
• Minimize building height Minimize building height
• Minimize number and size of clean rooms, particularly Minimize number and size of clean rooms, particularly
Class 100 rooms Class 100 rooms
• Minimize size of clean corridors and staging areas Minimize size of clean corridors and staging areas
19
Architecture & Layout Considerations Architecture & Layout Considerations
Minimize height of building to extent possible. Minimize height of building to extent possible.
Height increases cost due to: Height increases cost due to:
– Increase in amount of perimeter wall for a given total Increase in amount of perimeter wall for a given total
floor area floor area
– Increased load on the structure Increased load on the structure
• Heavier load on columns and footings Heavier load on columns and footings
– Additional hoisting of materials and extra time taken by Additional hoisting of materials and extra time taken by
operators to reach the higher floors operators to reach the higher floors
20
Architecture & Layout Considerations Architecture & Layout Considerations
• Thermal currents Thermal currents
• Unidirectional airflow shading Unidirectional airflow shading
21
GMP’s 21 CFR Part 211 21 CFR Part 211
– Subpart C Subpart C
-Buildings and Facilities Buildings and Facilities
• § 211.42 Design and construction features. § 211.42 Design and construction features.
• (a) Any building or buildings used (a) Any building or buildings used in the manufacture, processin in the manufacture, processing,
packing, or holding of a drug product shall be of packing, or holding of a drug product shall be of suitable size suitable size,
construction and location to fac construction and location to facilitate cleaning, maintenance, a ilitate cleaning, maintenance, and
proper operations. proper operations.
• (b) Any such building shall have (b) Any such building shall have adequate space adequate space for the orderly for the orderly
placement of equipment and materi placement of equipment and materials to prevent mixups between als to prevent mixups between
different components, drug produc different components, drug product containers, closures, labelin t containers, closures, labeling, in
–
process materials, or drug produc process materials, or drug products, and to prevent contaminatio ts, and to prevent contamination.
• The flow of components, drug pr The flow of components, drug product containers, closures, label oduct containers, closures, labeling,
in
-process materials, and drug prod process materials, and drug products through the building or ucts through the building or
buildings shall be buildings shall be designed to prevent contamination designed to prevent contamination.
• (c) Operations shall be performed within specifically defined (c) Operations shall be performed within specifically defined areas of areas of
adequate size adequate size.
22
Example Equipment Layout Example Equipment Layout
23
Architecture & Layout Considerations Architecture & Layout Considerations
• Room criteria sheets help to de Room criteria sheets help to define the requirements upfront (al fine the requirements upfront (also
referred to as Lab Cards) referred to as Lab Cards)
Room Name: Main Compounding Room
General Area: Compounding
Room no. 128
Structural
Hoist
Monorail
Floor pits (scales)
Operational Issues: Three fixed tanks, 100 L, 500 L, 1,000 L
wash down
Special material handling
Purified water Drop (3 use points)
Miscellaneous: Wall bumpers
Roof hatches
Armor plate on doors
Shelving
Storage cabinet
24
Architecture & Layout Considerations Architecture & Layout Considerations
Gowning rooms play a critical role in the facility layout. Gowning rooms play a critical role in the facility layout.
Cleanroom Cleanroom clothing: clothing:
• Designed to limit the rate of particle generation from the Designed to limit the rate of particle generation from the
person
• Designed to limit the rate of particle generation from the clean Designed to limit the rate of particle generation from the clean
room garment. room garment.
• In cleanrooms where contamination is not as important (e.g. In cleanrooms where contamination is not as important (e.g.
pharmaceutical areas and Class 100,000 areas), smock, cap and pharmaceutical areas and Class 100,000 areas), smock, cap and
shoe covers may be appropriate. shoe covers may be appropriate.
25
Architecture & Layout Considerations Architecture & Layout Considerations
Changing rooms: Changing rooms:
Two grades (levels) of changing rooms Two grades (levels) of changing rooms
• Low (standard) Low (standard)
– From normal clothing (street clothes) to factory From normal clothing (street clothes) to factory
(clean) clothing (clean) clothing
• High (standard) High (standard)
– From clean clothing to full coverage suit From clean clothing to full coverage suit
26
Architecture & Layout Considerations Architecture & Layout Considerations
Cleanroom clothing requirements are found in: Cleanroom clothing requirements are found in:
• IEST Recommended Practice RP IEST Recommended Practice RP
-CC
-003.2
• EU Guidelines EU Guidelines
27
Architecture & Layout Considerations Architecture & Layout Considerations
Cleanroom clothing: Cleanroom clothing:
• In cleanrooms where contamination is In cleanrooms where contamination is
critical, (e.g. Class 10,000 and Class 100 critical, (e.g. Class 10,000 and Class 100
areas), a full coverage coverall, hood, boots, areas), a full coverage coverall, hood, boots,
mask, gloves and goggles are worn. mask, gloves and goggles are worn.
28
Architecture & Layout Considerations Architecture & Layout Considerations
Gray zones: Service space or maintenance Gray zones: Service space or maintenance
space typically adjacent to the production space typically adjacent to the production
room
• Contains the majority of piping, valves, electrical Contains the majority of piping, valves, electrical
conduit and other utilities that support the conduit and other utilities that support the
manufacturing area. manufacturing area.
• Maintenance personnel have separate access to Maintenance personnel have separate access to
these areas, allowing less stringent gowning these areas, allowing less stringent gowning
requirements, and allows for maintenance requirements, and allows for maintenance
without shutting down or disrupting the without shutting down or disrupting the
manufacturing operation manufacturing operation
29
Example Layout Example Layout
Material
s Airlock
Change room/
Gowning Area
OVEN
PREPARATION
AREA
ASEPTIC CORRIDOR
ASEPTIC
FILLING
ROOM
AREA
30
Material & Personnel Flow Example Material & Personnel Flow Example
Materials
Airlock
Change room/
Exit
OVEN
PERSONNEL ENTRY
ASEPTIC CORRIDOR
ASEPTIC
FILLING
ROOM
AREA
GOWNING
AREA – IN
MATERIAL ENTRY
31
Desirable Desirable
Layout
Aseptic Core
Receiving Dock
Receiving Dept.
Incoming Materials
Wash and
Prep
Controlled Pharm Corridor
Final
Aseptic
Processing
Secondary Packaging
Shipping Dept.
Loading Dock
32
Less Desirable Layout Less Desirable Layout
Loading Dock
Shipping and
Receiving
Secondary
Packaging
Aseptic Core
Wash and
Prep
Final Aseptic
Processing
33
Materials of Construction & Materials of Construction &
Surface Finishes Surface Finishes
34
Materials of Construction & Materials of Construction &
Surface Finishes Surface Finishes
•
§ 211.42 Design and construction features. 211.42 Design and construction features.
• There shall be separate or defined areas for the There shall be separate or defined areas for the
firm’s operations to prevent contamination or firm’s operations to prevent contamination or
mixups as follows: as follows:
• (10) Aseptic processing, which includes as (10) Aseptic processing, which includes as
appropriate: appropriate:
• (I) Floors, walls, and ceilings of smooth, hard Floors, walls, and ceilings of smooth, hard
surfaces that are easily cleanable; surfaces that are easily cleanable;
35
Materials of Construction & Materials of Construction &
Surface Finishes Surface Finishes
• There is no such thing as FDA endorsed materials There is no such thing as FDA endorsed materials
• Surface finishes should be smooth, non Surface finishes should be smooth, non
-shedding, non shedding, non
–
porous, and resistant to sustaining microbial growth porous, and resistant to sustaining microbial growth
• Finishes must withstand repeated cleaning and Finishes must withstand repeated cleaning and
sanitization* without evidence of rust, or peeling paint. sanitization* without evidence of rust, or peeling paint.
*Cleaning and sanitization agents include *Cleaning and sanitization agents include
detergents and disinfectants, as well as hot WFI. detergents and disinfectants, as well as hot WFI.
• Stainless steel often used throughout the facility Stainless steel often used throughout the facility
because of its appearance, durability, smoothness, and because of its appearance, durability, smoothness, and
resistance to rust, peeling and shedding resistance to rust, peeling and shedding
36
Materials of Construction & Surface
Finishes
• Ledges, joints, and corners Ledges, joints, and corners difficult to reach should difficult to reach should be minimized be minimized
• Door hardware should be minimized Door hardware should be minimized
– Use proximity sensors wherever possible Use proximity sensors wherever possible
• A cleanroom cleanroom should be built airtight, where possible should be built airtight, where possible
• Internal surfaces smooth and suitable for cleaning Internal surfaces smooth and suitable for cleaning
• Surfaces must be resistant to impact Surfaces must be resistant to impact
• Joints should be free of openings that could harbor Joints should be free of openings that could harbor
dirt or microbes dirt or microbes
• Crack and crevice Crack and crevice
-free construction free construction
37
Materials of Construction & Surface
Finishes
• Concealed, sealed sprinklers should be used to avoid Concealed, sealed sprinklers should be used to avoid
communication between communication between cleanroom cleanroom and interstitial and interstitial
space
• Electrical outlets should be covered/sealed suitable Electrical outlets should be covered/sealed suitable
for washdown washdown service service
• Predetermined routes for removing/installing tanks Predetermined routes for removing/installing tanks
and other stationary equipment and other stationary equipment
– Removable wall panels often used to avoid tear Removable wall panels often used to avoid tear-out later. out later.
• Bumper guards on doors and corridors that are Bumper guards on doors and corridors that are
subjected to heavy equipment travel subjected to heavy equipment travel
38
Materials of Construction & Surface
Finishes
• Platforms typically stainless steel, including Platforms typically stainless steel, including
decking, stairs and support structure decking, stairs and support structure
• Stainless steel screens on HEPA filters Stainless steel screens on HEPA filters
• Stainless steel benches for gowning areas Stainless steel benches for gowning areas
• Recessed fire extinguishers with stainless steel Recessed fire extinguishers with stainless steel
frame
• All access panels stainless steel All access panels stainless steel
39
Materials of Construction & Surface Materials of Construction & Surface
Finishes Finishes
40
Materials of Construction & Surface
Finishes
Flooring: Flooring: Consider aesthetics, durability and cleanability Consider aesthetics, durability and cleanability
• Epoxy terrazzo: Epoxy terrazzo:
– hard, durable long hard, durable long-lasting surface with excellent chemical resistance lasting surface with excellent chemical resistance
– Very expensive to install Very expensive to install
• Epoxy Resin Systems: (e.g. Epoxy Resin Systems: (e.g. Stonhard Stonhard)
– Very popular in the Pharmaceutical industry Very popular in the Pharmaceutical industry
– Easier to install than Terrazzo Easier to install than Terrazzo
– Moderate durability and chemical resistance Moderate durability and chemical resistance
– Moderate price Moderate price
• Welded Vinyl/PVC Sheet (e.g. Welded Vinyl/PVC Sheet (e.g. Mipolam Mipolam)
– Durability an issue in high Durability an issue in high
-traffic areas traffic areas
– Often used with identical wall system for matching Often used with identical wall system for matching
41
Materials of Construction & Surface
Finishes
Mipolam Mipolam
42
Materials of Construction & Surface Finishes
Typical seamless epoxy resin flooring system Typical seamless epoxy resin flooring system
1.Polyurethane
Coating
(Gloss or Matte)
2.Epoxy Clear Coat
3.Broadcast
4.Epoxy Coat
5.Heavy Duty Mortar
6.CornerCrete Slurry
Primer
7.Substrate
43
• Epoxy terrazzo: Epoxy terrazzo:
– hard, durable long hard, durable longlasting
surface with lasting surface with
excellent chemical excellent chemical
resistance resistance
– Very expensive to Very expensive to
install install
Flooring Flooring
44
Radius cove base typically Radius cove base
used
Materials of Construction & Surface
Finishes
Radius cove base in modular
construction
45
Materials of Construction & Surface
Finishes
Flooring/drains Flooring/drains
• Drains should be avoided in Class 100 Drains should be avoided in Class 100
through Class 10,000 rooms. through Class 10,000 rooms.
• Acceptable in Class 100,000 rooms Acceptable in Class 100,000 rooms
46
Materials of Construction & Surface
Finishes
Ceiling Systems Ceiling Systems
• Same issues are important: aesthetics, durability and cleanabili Same issues are important: aesthetics, durability and cleanability
• Gypsum Wall Gypsum Wall-board (GWB) with finish coat of epoxy paint board (GWB) with finish coat of epoxy paint
– Pro’s:
• Flexible, easy to use Flexible, easy to use
• Most common in the industry Most common in the industry
– Con’s:
• Restricts future above Restricts future above
-ceiling access ceiling access
• Requires careful coordination with other trades during Requires careful coordination with other trades during
construction. (e.g. piping penetr construction. (e.g. piping penetrations, HVAC diffusers, light ations, HVAC diffusers, light
fixtures, filter housings). fixtures, filter housings).
47
Materials of Construction & Surface Finishes
Ceiling Systems Ceiling Systems
• Lay
-in grid ceilings ( in grid ceilings (http:// http://www.cleanroomeng.com/products_ceiling.cfm www.cleanroomeng.com/products_ceiling.cfm)
• Use gasketed clean Use gasketed clean
-room tiles room tiles
• Seams and joints still a potenti Seams and joints still a potential breeding ground for bacteria al breeding ground for bacteria
48
Materials of Construction & Surface Finishes
Light fixtures Light fixtures
• Lay
-in grid ceilings in grid ceilings
– sealed units with stainless trim sealed units with stainless trim
• Teardrop Teardrop
49
For Class 100 through For Class 100 through
Class 10,000 (Grade A and Class 10,000 (Grade A and
Grade B areas), radius Grade B areas), radius
coves are often used in the coves are often used in the
ceiling to wall junction as well ceiling to wall junction as well
Materials of Construction & Surface Finishes
Radius cove where ceiling
meets wall. (modular
construction shown).
50
Materials of Construction & Surface Finishes
Wall systems Wall systems
• Consider aesthetics, durability and cleanability Consider aesthetics, durability and cleanability
• Gypsum Wall Gypsum Wall
-board (GWB) with epoxy paint board (GWB) with epoxy paint
finish
• Concrete Block/concrete block with plaster Concrete Block/concrete block with plaster
• Epoxy Resin walls Epoxy Resin walls
• Welded sheet PVC (e.g. Welded sheet PVC (e.g. Mipolam Mipolam)
– Typically used in higher class areas (e.g. Class 100) Typically used in higher class areas (e.g. Class 100)
51
Materials of Construction & Surface Finishes
Wall systems Wall systems
(Vision Panels) (Vision Panels)
• Flush with the wall to Flush with the wall to
eliminate ledges eliminate ledges
• Often Stainless frame, Often Stainless frame,
double pane double pane
• Free of gaps along the Free of gaps along the
perimeter perimeter
52
Materials of Construction & Surface Finishes
Cleanroom Doors
Full glass or half glass architectural aluminum doors are available with a
variety of hardware options, including pivot hinges, surface mounted closers,
concealed closers, panic hardware, and locksets.
53
Materials of Construction & Surface Finishes
Steel Doors
Glazed or flush steel doors integrate into the aluminum door frames utilizing butt
hinges at the jamb connection.
54
Materials of Construction & Surface Finishes
Electric Sliding
Sliding doors easily integrate into wall systems and are available in any size with
a variety of hardware available including push button entries and motion sensors.
55
Materials of Construction & Surface Finishes
High
-Speed Roll Speed Roll-Up Doors Up Doors
Roll Up Doors offer advantages over swinging doors, sliding doors, and strip
curtains. Doors can be created up to 18′ x 18′. Suitable for Class 100,000 to Class
10,000 applications. The door opens and closes quickly reducing the time the
cleanroom interior is exposed.
56
Materials of Construction & Surface Finishes
Glazing Glazing
Windows and doors can be glazed with a variety of options as specified by the
project requirements. One can choose from tempered glass, tinted glass, Lexan,
acrylic, static dissipative, film-covered glass or solid panels
57
Materials of Construction & Surface Finishes
AIR SHOWERS
Chambers located between the clean room and an outside environment that
remove particulate contamination from clean room garments as personnel pass
through. The chambers may include HEPA filters, interlocking doors, a recirculating
air system, and air nozzles in various patterns through which filtered
air is blown onto the personnel in the shower. The high-velocity air is moved over
the worker, removing particulate contamination from the worker’s garments.
58
Materials of Construction & Surface Finishes
Modular Modular Cleanroom Cleanroom Facilities Facilities
Self-contained facilities built off contained facilities built off-site under controlled site under controlled
conditions, then delivered and integrated into the final conditions, then delivered and integrated into the final
point of use location with the minimal amount of point of use location with the minimal amount of
reassembly. reassembly.
• Very popular alternative. Very popular alternative.
Three ISPE Facility of the Year Awards were given to Three ISPE Facility of the Year Awards were given to
firms that utilized the modular concept. firms that utilized the modular concept.
– Novo
-Nordisk, Denmark Nordisk, Denmark
– Baxter Biopharma Biopharma Solutions, Bloomington, Indiana Solutions, Bloomington, Indiana
– GSK
59
Modular Clean Modular Clean
-room Facilities room Facilities
• Pre
-engineered, pre engineered, pre
-fabricated walls and top decks that fabricated walls and top decks that
can support weight of air handling equipment, ceilings can support weight of air handling equipment, ceilings
and filters. and filters.
• Can assemble relatively quickly in the field. Can assemble relatively quickly in the field.
• One source for virtually everything for the One source for virtually everything for the cleanroom cleanroom,
airhandling airhandling, HEPA filters, wall panels, lighting, , HEPA filters, wall panels, lighting,
windows, doors, instrumentation and controls, all built windows, doors, instrumentation and controls, all built
into the package. into the package.
• Wall and ceiling systems typically of metal panels with Wall and ceiling systems typically of metal panels with
special coatings that resist cleaning agents and give special coatings that resist cleaning agents and give
aesthetically pleasing look aesthetically pleasing look.
• Can opt for PVC finished panels or stainless steel finish Can opt for PVC finished panels or stainless steel finish
panels also. panels also.
Materials of Construction & Surface Finishes
60
Materials of Construction & Surface Finishes
Modular Clean Modular Clean
-room Facilities room Facilities
– Sample Manufacturer Sample Manufacturer
PharmaWall System from PortaFab.
“Designed specifically for pharmaceutical
and biotechnology facilities, the
PharmaWall System utilizes a patent
pending “Z” clip design that integrates
with our 1/2″ (12.7 mm) thick panels to be
hung off a metal stud framework or an
existing wall. The non-progressive system
allows for the easy removal of panels.
By connecting to a metal stud framework
you no longer have to be limited with the
depth of your utility chases. All piping,
mechanical and electrical processes can be
easily integrated within 3-5/8″, 6″, 12″ or
18″ cavities without having to create a
“double” wall out of two free-standing
partition walls.”
61
Materials of Construction & Surface Finishes
Modular Clean Modular Clean
-room Facilities room Facilities
– Sample Manufacturer Sample Manufacturer
PharmaWall System from PortaFab.
Process & Utility Integration
“Piping penetrations and enclosures can be
easily created by utilizing the PharmaWall
system design. The radius cove details and
cleanable surfaces meet the requirements for a
clean space and allow for future piping
expansion capability.
Process service panels are easily integrated into
the PharmaWall system in a recessed manner
that minimizes ledges and joints. The panels
can be designed to allow maintenance access
and provide for future piping expansion.
The PharmaSystem’s modular design also
allows cleanroom designers and contractors the
ability to make field modifications during the
installation process. This flexibility provides
for a number of potential design cost savings.”
62
Materials of Construction & Surface Finishes
Modular Clean Room Facilities: Modular Clean Room Facilities:
• Reasons why modular construction may be used: Reasons why modular construction may be used:
– Compressed construction time Compressed construction time
– Flexibility for future changes Flexibility for future changes
– Other reasons: Other reasons:
• Overcome local skill shortages Overcome local skill shortages
• Reduced weight Reduced weight
• Reduce number of contractors used during construction Reduce number of contractors used during construction
– Rely on the modular firm? Rely on the modular firm?
• Pre
-qualification testing and customer inspection of the qualification testing and customer inspection of the
facilities are completed before shipment to assure facilities are completed before shipment to assure
compliance. compliance.
• Increased safety (Transferred to vendor?) Increased safety (Transferred to vendor?)
63
Materials of Construction & Surface Finishes
Modular Clean Room Facilities Modular Clean Room Facilities
Impediments to use of modular construction: Impediments to use of modular construction:
• Generally more expensive than the traditional “stick Generally more expensive than the traditional “stickbuilt”
clean room built” clean room
• Increased engineering costs Increased engineering costs
• Early design freeze which may reduce flexibility of scope Early design freeze which may reduce flexibility of scope
• Complicated interface (coordination) issues Complicated interface (coordination) issues
• Absence of a robust economic advantage Absence of a robust economic advantage
• Must complete flooring, sprinklers, and other utilities in Must complete flooring, sprinklers, and other utilities in
the field (i.e.: water, electric, specialty gases, etc) the field (i.e.: water, electric, specialty gases, etc)
64
Room Pressurization Room Pressurization
• Both US and EU requires that rooms of higher grade must be Both US and EU requires that rooms of higher grade must be
at higher pressure levels. at higher pressure levels.
• Typically 0.05” water column Typically 0.05” water column difference between classes. difference between classes.
• Ensures air flows from cleaner ar Ensures air flows from cleaner areas to dirtier areas. Class eas to dirtier areas. Class
100 filling rooms always have the highest pressure. 100 filling rooms always have the highest pressure.
• Class 100 fill rooms will sometimes have regions that are class Class 100 fill rooms will sometimes have regions that are class
10,000 (there is no requirement 10,000 (there is no requirement to have the entire room at to have the entire room at
class 100) however the room is still considered a class 100 class 100) however the room is still considered a class 100
room in terms of pressure levels. room in terms of pressure levels.
External Areas
Streets, Offices, Restaurant
Raw
Materials People
Remove
Outers
Sterilize
Change Change Remove Outers
Container/Closures
Compounding
Critical Processing Area
E.g.: Point of Fill
Transition Zone
Brings people, materials, etc. from
external areas to the manufacturing
areas in a “controlled” manner
Clean Area
Provides a protective
envelope to minimize the
challenge to the Critical Areas
66
FILLING
ROOM CLASS
100
REGION
CLASS 10,000
BACKGROUND
ASEPTIC CORRIDOR
CLASS 10,000
CLASS 100,000
CORRIDOR
CLASS 100,000
CORRIDOR
CLASS 100,000
PREPARATION AREA
PHARMACEUTICAL
AREA
PHARMACEUTICAL AREA
LOCKER ROOM – ENTRY AREA
Classification Levels within an Aseptic Facility
Nested Manufacturing Zones (Five Zones)
67
Clean Room Pressurization – Example
Sample Sterile
Manufacturing Suite Airflow
Direction
Building
Corridor
0.00”
Prep
Room
Class
100,000
0.05”
Aseptic
Processing
Class 10,000
0.10”
Filling Room
Class 100
Highest Pressure
0.15”
68
Another
example
of
cascading
room
pressures
69
Example of an actual facility layout