ISCOMS (immune stimulatory complexes):
Some plant materials have been evaluated, including the soap-like saponins from Quilaja saponaria and the highly purified Quil A extract obtained from saponins which induce the production of cytokines. In common with all saponins these materials have hemolytic activity that limits their direct use in humans although it is used in veterinary medicine. However, combination of Quil A with cholesterol, phospholipids, and antigens forms a human-compatible adjuvant called immunostimulatory complexes (ISCOMs). ISCOMs have an interesting range of adjuvantactivities resulting in an increased Th1 response.
Hypothetical transport route(s) across the intestinal epithelium of antigens incorporated into ISCOMs or delivered with ISCOMATRIX.
- Uptake and transport of ISCOMs by M cells in the Peyer’s patches;
- Uptake and intracellular transport of ISCOMs by enterocytes;
- Paracellular transport of antigen as a result of surfactant-like effects of ISCOMATRIX on the epithelial integrity.
- ISCOMs are 40 nm large particles made up of saponins (Quil A), lipids, cholesterol and antigen, held together by hydrophobic interactions between the first three components.
- Cholesterol is the ligand that binds to saponin forming 12 nm rings.
- These rings are fixed together by lipids to form the spherical nanoparticles. Hydrophobic or amphipathic antigens can be incorporated into this complex.
- They are versatile and flexible delivery systems with increased efficiency of antigen presentation to B cells and uptake by the APC. vaccines are potent inducer of both humoral and cellular (CD4+ and CD8+ T-cell) immune responses.
- The use of saponins in ISCOMs-based vaccines retains the adjuvant activity of the saponin component but with a reduced toxicity.
- The ISCOMATRIX adjuvant is identical to ISCOMs except that it does not contain antigen. This adjuvant can be mixed with antigens and has some of the advantages of ISCOMs such as the preferential targeting of antigen to APC.
- However, the response obtained differed from that of ISCOMs vaccination in that the ISCOMATRIX induced a Th2-like response, whereas the ISCOMs-based vaccine induced a mixed Th1/Th2 response.
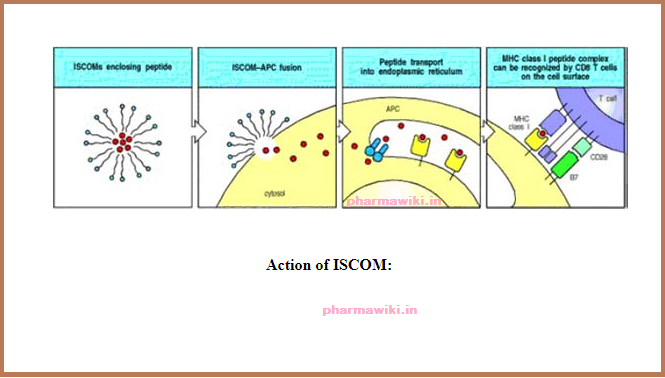
Action of ISCOMs
ISCOMs (immune stimulatory complexes) are lipid micelles that will fuse with cell membranes. Peptides trapped in ISCOMs can be delivered to the cytosol of an antigenpresenting cell (APC), allowing the peptide to be transported into the endoplasmic reticulum, where it can be bound by newly synthesized MHC class I molecules and hence transported to the cell surface as peptide:MHC class I complexes. This is a possible means for delivering vaccine peptides to activate CD8 cytotoxic T cells. ISCOMs can also be used to deliver proteins to the cytosol of other types of cell, where they can be processed and presented as though they were a protein produced by the cell.
Liposomes:
- Liposomes are synthetic spheres comprised by lipid bilayers that can encapsulate antigens and act as both a vaccine delivery vehicle and adjuvant.
- The potency of liposomes depends on the number of lipid layers, electric charge, composition and method of preparation
- Depot formation at the site of injection and efficient presentation of antigens to macrophages.
- Both humoral and cell-mediated immune responses have been elicited by liposomes.
- Immunostimulators such as LPS and MDP when encapsulated within liposomes show enhanced adjuvanticity with reduced side effects.
- However, phospholipid liposomes have certain limitations such as sensitivity to host phospholipases, instability on storage, high cost of manufacture and difficulty in scale up of production.
- To overcome these problems non-phospholipid liposomes are developed. These non-phospholipid liposome vesicles, composed of dioxyethylenecetyl ether, cholesterol and oleic acid, were evaluated with human vaccine antigens (tetanus and diphtheria toxoids) in rabbits and mice.
- Tetanus and diphtheria toxoids encapsulated in or mixed with these liposomes elicited antitoxin levels similar to those elicited by antigens given with FCA or adsorbed onto aluminum adjuvants.
- Recent results have suggested that, by choosing lipid components for liposomes, surface-coupled liposomal antigens might be applicable for the development of tumor vaccines to present tumor antigens to antigen-presenting cells (APC) and induce antitumor responses.
- Liposomes have been used to deliver vaccines and have been observed to have immunostimulant activity. When administered orally liposomes with encapsulated antigens have been claimed to provide protection from the gastric proteolytic enzymes. Liposomes also have potential as mucosal delivery systems since not only are they immunogenic in their own right but physical association of the antigen with the liposomal structure is not a requirement for intranasal immunostimulation.
- Simple mixtures of liposomes with other agents such as chitosan have potential for nasal delivery and have facilitated enhanced responses to vaccines administered orally.
- Cochleate systems consisting of calcium-precipitated protein–phospholipids complexes are stable solid sheets that roll up into a spiral with no internal aqueous space, and the calcium ions bridge adjacent sheets. Oral administration of vaccines in cochlear delivery systems has been shown to induce strong long-lasting circulation and mucosal antibody responses with long-term immunological memory to influenza glycoproteins.
Virosomes:
Another type of liposomes, referred to as virosomes, contain a membrane bound hemagglutinin and neuraminidase derived from influenza virus, and serve to amplify fusogenic activity and therefore facilitate the uptake into APCs and induce a natural antigen-processing pathway.
Virosomes are viral glycoproteins encapsulated in lipid vesicles, which have been shown to be effective as experimental vaccines delivered by both mucosal and systemic routes.
Viruses and their surface glycoproteins have a high affinity for receptors on mucosal surfaces, especially along the respiratory tract.
EXAMPLE:-
IRIV – contains (Haemaglutinin from influenza virus) which intercalates in to phospholipid bilayer & acts to stabilize liposomal base preventing fusion with the liposome.
- Immune potentiation of IRIV is due to HA1 (SUBUNIT) which binds to sialic acid residues (which are present on the surface of macrophages & other immune competent cells, finally resulting in endocytic uptake.
- Upon exposure to low (pH=5)- The HA2 SUBUNIT undergoes conformational change exposing the fusogenic peptide resulting in the fusion of IRIV and endosomal membrane