Contents of the powerpoint on Multiple Emulsions include:
INTRODUCTION
FORMULATION OF MULTIPLE EMULSIONS
PREPARATION OF MULTIPLE EMULSIONS
CHARACTERISATIONOFMULTIPLEEMULSIONS
STABILITY OF MULTIPLE EMULSIONS
STABILITY ASSESSMENT STUDIES
DRUG RELEASE FROM MULTIPLE EMULSIONS
BIOAVAILABILITY
APPLICATIONS
CONCLUSION
REFERENCES
Download the powerpoint by liking us on Facebook
[like-gate][/like-gate]
[button url=”http://pharmawiki.in/?attachment_id=3409″ style=”glass” background=”#1782f9″ color=”#ffffff” size=”4″ center=”yes” icon=”icon: download”]Download PPT here[/button]
You can also download the PDF onMultiple Emulsions by clicking here
[button url=”http://pharmawiki.in/?attachment_id=3416″ style=”glass” background=”#1782f9″ color=”#ffffff” size=”4″ center=”yes” icon=”icon: download”]Download PDF here[/button]
Let’s delve into each aspect of multiple emulsions in detail.
Introduction to Multiple Emulsions
Multiple emulsions, also known as “W/O/W” or water-in-oil-in-water emulsions, are complex systems that involve the dispersion of both oil and water phases within one another. These emulsions are characterized by a double-layered structure, where an inner water phase is surrounded by an oil phase, which is, in turn, encapsulated by another outer water phase. Multiple emulsions have garnered significant interest in various industries due to their unique properties, including the ability to encapsulate and deliver both hydrophilic (water-soluble) and lipophilic (oil-soluble) compounds simultaneously. This makes them particularly valuable in pharmaceuticals, cosmetics, and food products, where controlled release and improved stability are critical.
Formulation of Multiple Emulsions
The formulation of multiple emulsions involves careful consideration of the specific objectives and the desired properties of the emulsion. The main components in multiple emulsion formulation include:
Primary Emulsion (W/O): This innermost phase typically consists of water-soluble compounds or hydrophilic drugs. It forms the core of the multiple emulsion.
W/O Interface: An intermediate layer contains emulsifying agents, stabilizers, or polymers, which help prevent phase separation between the inner and outer phases. The interface plays a crucial role in maintaining the integrity of the multiple emulsion structure.
Secondary Emulsion (O/W): The outer phase is usually composed of oil, which may contain lipophilic compounds or drugs. This phase surrounds the primary emulsion, forming the final double-layered structure.
Preparation of Multiple Emulsions
Multiple emulsions can be prepared using various techniques, depending on the desired structure and characteristics. Two common methods include:
Two-Step Emulsification: This approach involves creating the primary emulsion first, which is typically a water-in-oil (W/O) emulsion. Then, the outer aqueous phase is added to form the final water-in-oil-in-water (W/O/W) multiple emulsion. This method allows for precise control over the composition of both the inner and outer phases.
Phase Inversion Method: The phase inversion method exploits changes in temperature or the addition of co-surfactants to induce a phase inversion between the primary emulsion (W/O) and the secondary emulsion (O/W). This technique can lead to the formation of multiple emulsions with different properties.
Characterization of Multiple Emulsions
Understanding and controlling the properties of multiple emulsions is crucial for optimizing their performance. Various characterization techniques are used to assess their structure, stability, and physical properties:
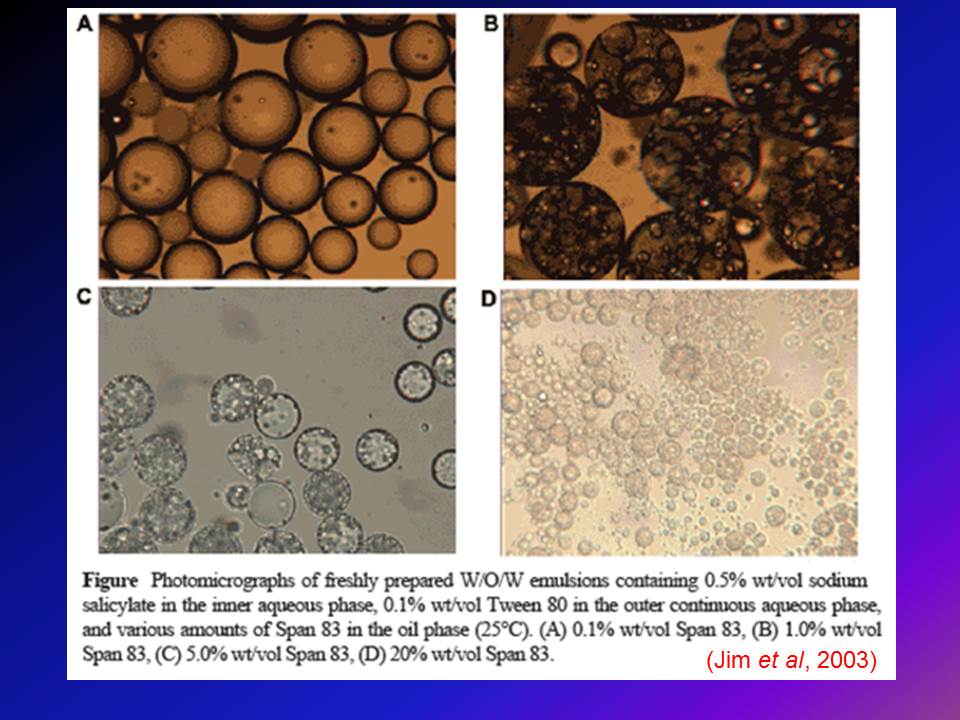
Microscopy: Optical and electron microscopy are employed to visualize the internal structure of multiple emulsions. This helps in observing the distribution of droplets within the emulsion.
Particle Size Analysis: Determining the size distribution of droplets in the emulsion is essential for assessing stability and predicting behavior. Techniques like dynamic light scattering (DLS) or laser diffraction are commonly used for this purpose.
Rheology: Rheological measurements help in understanding the viscosity and flow behavior of multiple emulsions. This information is critical for applications such as cosmetics and food products.
Zeta Potential: Zeta potential measurements provide insights into the surface charge of droplets. This parameter affects stability, as droplets with higher or lower surface charges may repel or attract each other, influencing aggregation and coalescence.
Stability of Multiple Emulsions
Ensuring the long-term stability of multiple emulsions is a significant challenge. Several factors can impact stability, and they need to be carefully addressed:
Ostwald Ripening: This phenomenon involves the continuous growth of larger droplets at the expense of smaller ones. It can lead to instability by causing changes in the size distribution of droplets.
Flocculation and Creaming: Flocculation refers to the aggregation or clustering of droplets. Creaming occurs when droplets migrate to the top or bottom of the emulsion due to density differences. Both can lead to phase separation.
Coalescence: Coalescence involves the merging of neighboring droplets, which can result in the formation of larger droplets and eventual phase separation.
Stability Assessment Studies
To address the challenges of stability in multiple emulsions, various stability assessment studies are conducted:
Accelerated Stability Testing: Multiple emulsions are subjected to extreme conditions, such as high temperatures or freeze-thaw cycles, to predict their long-term stability under harsh environmental conditions.
Freeze-Thaw Cycling: This test simulates temperature fluctuations that may occur during storage or transportation, helping to evaluate the emulsion’s resilience to thermal stress.
Centrifugation: Centrifugation is used to assess phase separation under force. It helps determine the emulsion’s stability when subjected to mechanical stress.
Visual Inspection: Regular visual inspection is essential for monitoring changes in the emulsion’s appearance, including color, clarity, and phase separation. Any signs of instability need to be addressed promptly.
Drug Release from Multiple Emulsions
Multiple emulsions find significant applications in controlled drug release. Several factors influence drug release from these emulsions:
Emulsion Composition: The choice of whether the drug is placed in the inner (W/O) or outer (O/W) phase impacts its release. Hydrophilic drugs are often placed in the inner phase, while lipophilic drugs are incorporated into the outer phase.
Emulsifier Type: The type and concentration of emulsifiers can significantly affect drug solubility within the emulsion. Proper selection is essential for achieving the desired release profile.
Drug Loading: The concentration of the drug in the emulsion can be adjusted to control the release rate. Higher drug concentrations typically result in faster release.
External Phase Viscosity: Altering the viscosity of the outer (O/W) phase can influence drug release kinetics. Higher viscosity can slow down drug diffusion.
Bioavailability
In pharmaceutical applications, the bioavailability of drugs delivered via multiple emulsions is of utmost importance. Several factors can impact bioavailability:
Droplet Size: Smaller droplets provide a larger surface area for drug absorption, potentially enhancing bioavailability.
Emulsifier Choice: The type and concentration of emulsifiers can affect drug solubility and stability within the emulsion. Proper selection is crucial for optimizing bioavailability.
Formulation: Adjusting the ratio of the oil to water phases can influence drug release and absorption. Finding the right balance is critical.
Patient Factors: Individual variations in physiology, metabolism, and gastrointestinal (GI) tract conditions can influence drug absorption and, consequently, bioavailability.
In conclusion, multiple emulsions are versatile systems with wide-ranging applications. Understanding their formulation, preparation, characterization, stability assessment, drug release mechanisms, and bioavailability considerations is essential for designing effective drug delivery systems and optimizing product performance. Researchers and formulators continue to explore and innovate in this field to harness the potential of multiple emulsions for improved therapeutic outcomes and product development in various industries.