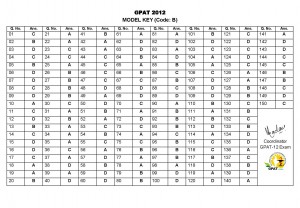
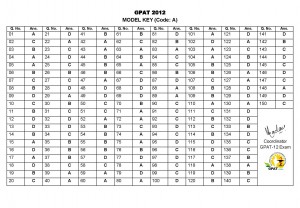
The Parliamentary Standing Committee on Health and Family Welfare also pointed to serious lapses and irregularities on the approvals of new drugs and pointed out that 33 such drugs were approved without conducting clinical trials on Indian patients.
The Committee said scrutiny of 42 drugs picked up randomly involving grant of drug approvals in utter disregard of regulatory procedures and violation of rules and pointed out to files of approval of three controversial drugs (pefloxacin, lomefloxacin and sparfloxacin) found missing and untraceable.
These drugs were either never marketed or withdrawn in the US, Canada, Britain, Australia and other countries.
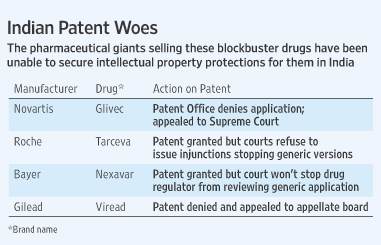
Citing the example of Deanxit, the Panel pointed out that the drug continued to be prohibited for sale and use in Denmark, the country of its origin, and thus permission to import and market it in India was given unlawfully.The panel cited another example of Letrozole by Novartis, used as an anti-cancer drugs used only in post-menopausal women, is used only in India where it is permitted for use in female infertility.
329 Banned Drugs List in India – PDF Full Download – 2018 – Latest News
List of Drugs Banned in India
A. Single drug preparations (or combinations of)
1. Amidopyrine
2. Phenacetin
3. Nialamide
4. Methaqualone
5. Methapyriline (and its salts)
6. Practolol
7. Penicillin skin/eye ointment
8. Tetracycline/Oxytetracyline/Demeclocycline liquid oral preparations.
9. Chloral hydrate
10. Dover’s powder and Dover’s powder tablets I.P.
11. Chloroform exceeding 0.5% w/w or v/v in pharmaceutical preparations.
12. Mepacrine HCl (Quinacrine and its salts) in any dosage form for use for female sterilization
or contraception.
13. Fenfluramine
14. Dexfenfluramine
15. Terfenadine
16. Astemizole
17. Phenformin
18. Rofecoxib
19. Valdecoxib
20. Rosiglitazone
21. Nimesulide Formulatios In Children Below The Age Of 12 years
22. Cisapride
23. Rimonabant
24. Phenyl Propanolamine
25. Human Placenta Extract in topical application for wound healing and injection for pelvic
inflammatory diseases.
26. Sibutramine
27. R-Sibutramine
28. Gatifloxacin
29. Tegaserod
B. Fixed dose combination with any other drug
1. Corticosteroids with any other drug for internal use.
2. Chloramphenicol with any other drug for internal use.
3. Sodium bromide/chloral hydrate with other drugs.
4. Ergot with any drug except preparations containing ergotamine, caffeine, analgesics,
antihistamines for treatment of migraine.
5. Anabolic steroids with other drugs.
6. Metoclopramide with other drugs (except with aspirin/paracetamol).
7. Pectin and/or kaolin with any drug which is systematically absorbed from g.i. tract, except
for combination of pectin and/or kaolin with drugs not systematically absorbed.
8. Hydroxyquinolines with any other drug except in preparations for external use.
9. Oxyphenbutazone or phenylbutazone with any other drug.
10. Dextropropoxyphene with any other drug except antispasmodics and/or NSAIDs.
11. Analgin (metamizol) with any other drug.
list of drugs banned in India PDF – 2018
C. Fixed dose drug combinations of
1. Penicillins with Sulfonamides.
2. Tetracyclines with Vitamin C
3. Antitubercular drugs with Vitamins (except Isoniazid with Pyridoxine HCl).
4. Vitamins with Analgesics/Antiinflammatory drugs.
5. Vitamins with Tranquillisers.
6. Atropine and Analgesic-antipyretics.
7. Yohimbine and Strychnine with Testosterone and Vitamins.
8. Strychnine and Caffeine in tonics.
9. Iron with Strychnine, Arsenic and Yohimbine.
10. Antihistaminics with Antidiarrhoeals.
11. More than one Antihistamine in the same preparation.
12. Sedatives/Hypnotics/Anxiolytics with Analgesic-antipyretics.
13. H2 receptor antagonists with Antacids (except those combinations approved by Drugs
Controller, India).
14. Anthelmintics (except Piperazine) with a Cathartic/Purgative.
15. Salbutamol (or any other bronchodilator) with centrally acting Antitussive and/or an
Antihistamine.
16. Centrally acting Antitussives with Antihistamines having atropine like activity in
expectorants.
17. Centrally acting Antitussive and/or Antihistamine in preparations for cough associated with
asthma.
18. Laxative and/or antispasmodic drugs in enzyme preparations.
19. Glycerophosphates and/or other phosphates and/or CNS stimulant in liquid oral tonics.
20. Estrogen and Progestin (other than oral contraceptives) containing per tablet Estrogen more
than 50 ug ethinylestradiol (or equivalent) and progestin more than 3 mg of norethisterone
acetate (or equivalent) and, all fixed dose combination injectable preparations containing
synthetic estrogen and progesterone.
21. Ethambutol with Isoniazid, except in the following daily doses:
Isoniazid 200 mg + Ethambutol 600 mg or
Isoniaizd 300 mg + Ethambutol 800 mg
22. Pyrazinamide with other antitubercular drugs, except that which provide the following daily
doses.
Rifampicin 450 to 600 mg
Isoniazid 300 to 400 mg
Pyrazinamide 1000 to 1500 mg
23. Essential oils with Alcohol having percentage higher than 20% proof (except preparations
given in the I.P.).
24. Liquid oral tonic preparations containing alcohol more than 20% proof.
25. Streptomycin with penicillin in parenteral preparation.
26. Antidiarrhoeals containing adsorbants like kaolin, pectin, attapulgite, activated charcoal etc.
27. Antidiarrhoeals containing phthalylsulfathiazole, succinyl sulfathiazole, sulfaguanidine,
neomycin, streptomycin, dihydrostreptomycin.
28. Antidiarrhoeal formulations for pediatric use containing diphenoxylate, loperamide, atropine,
hyoscyamine, halogenated hydroxyquionolines.
29. Antidiarrhoeals with electrolytes.
30. Fixed dose combinations of haemoglobin in any form.
31. Pancreatine or pancrelipase containing amylase, protease and lipase with any other enzyme.
32. Oral rehydration salts other than those conforming to the following parameters:
a) Oral rehydration salts on reconstitution to one litre shall contain: sodium-50 to 90 mM;
total osmolarity-240 to 290 mOsm; dextrose: sodium molar ratio-not less than 1:1 and not
more than 3:1.
b) Cereal based ORS on reconstitution to one litre shall contain: total osmolarity not more
than 2900 mOsm. Precooked rice equivalent to not less than 50 g and not more than 80 g
as total replacement of dextrose.
c) ORS may contain amino acids in addition to ORS conforming to the parameters
specified above and labeled with the indication for “Adult Choleratic Diarrhoea” only.
d) ORS shall not contain mono or polysaccharides or saccharin sweetening agent.
33. A drug, standards of which are prescribed in the 2nd schedule to Drugs and Cosmetics Act
with an Ayurvedic Siddha or Unani drug.
34. Vitamin B1, Vit B6 and Vit B12 for human use.
35. Diazepam with diphenhydramine HCl.
36. Nitrofurantoin with Trimethoprim.
37. Phenobarbitone with any antiasthmatic drug, or with hyoscine and/or Hyoscyamine, or
ergotamine and/or belladonna.
38. Haloperidol with any anticholinergic agent including propantheline Br.
39. Nalidixic acid with any antiamoebic including metronidazole.
40. Loperamide with furazolidone.
41. Cyproheptadine with lysine or peptone.
42. Diazepam and Diphenhyhydramine Hydrochloride.