VACCINE ADJUVANTS:
In immunology an”adjuvant is an agent that may stimulate the immune system and increase the response to a vaccine, without having any specific antigenic effect in itself”. The word “adjuvant” comes from the Latin word adjuvare, meaning to help or aid.” An immunologic adjuvant is defined as any substance that acts to accelerate, prolong, or enhance antigen-specific immune responses when used in combination with specific vaccine antigen.”
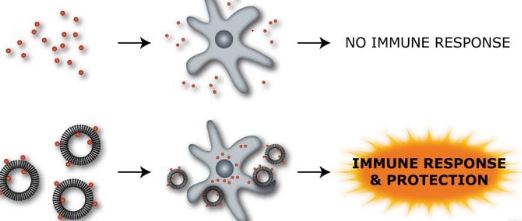
Adjuvants in immunology are often used to modify or augment the effects of a vaccine by stimulating the immune system to respond to the vaccine more vigorously, and thus providing increased immunity to a particular disease. Adjuvants accomplish this task by mimicking specific sets of evolutionarily conserved molecules which include liposomes, lipo polysaccharides (LPS), molecular cages for antigen, components of bacterial cell walls, and endocytosed nucleic acids such as double-stranded RNA (dsRNA), single-stranded DNA (ssDNA), and unmethylated CpG dinucleotide-containing DNA. Because immune systems have evolved to recognize these specific antigenic moieties, the presence of adjuvant in conjunction with the vaccine can greatly increase the innate immune response to the antigen by augmenting the activities of dendritic cells (DCs), lymphocytes, and macrophages by mimicking a natural infection Furthermore, because adjuvants are attenuated beyond any function of virulence, they pose little or no independent threat to a host organism.
Advantages of Adjuvant Action:
• Increase potency of weak small synthetic peptides.
• Enhance speed, vigor and persistence of immune response.
• Increase immune response in immunologically immature, immunosuppressed or senescent groups.
• Select for modulate cell mediated immunity (Major histocompatibility Class I) or humoral (Major histocompatibility Class II) responses.
• Modulate Ab avidity, specificity
• Reducing the dose of an antigen required for a response
• Increasing safety and reducing production costs.
• Decrease the amount of Ag in combination vaccine, reduce the likelihood of Ag competition.
SIDE EFFECTS OF VACCINE ADJUVANTS:
- Toxicity and adjuvant activity must be balanced to obtain maximum immune stimulation with minimal adverse effects.
- Majority of adjuvants produce some effects
like:-
Local reactions
The inflammatory response
Local pain and tissue lysis
Granulomas and
hypersensitivity reactions
Systemic effects
Real
& Theoretical risks of Vaccine Adjutants:
- Local acute or chronic inflammation with
formation of painful abscess, persistent nodules, ulcers or draining
lymphadenopathy.
- Induction of influenza like illness, with
fever, malaise, myalgia arthralgic or headache.
- Systemic clinical toxicity to tissues or
organs.
- Induction of hypersensitivity to host tissue,
producing autoimmune arthritis, amyloidosis, anterior uveitis.
- Cross reactions with human antigens, such as
glomerular basement membranes or neurolemma, causing glomerulo-nephritis or
meningoencephalitis.
- Sensitization to tuberculin or other skin
test antigens.
- Carcinogenesis; Teratogenesis; Abortogenesis
- Dissemination of live vector within the host
to cause disease; spread of the vector to the environment and other persons.
SAFETY EVALUATION OF VACCINE ADJUVANTS:-
It is a generally
accepted principle that toxicity and adjuvant activity must be balanced to
obtain maximum immune stimulation with minimal adverse effects.However, the
actual acceptance level for adverse reactions depends on whether the adjuvant
is intended for use in human or veterinary vaccines. For veterinary
applications the acceptance level depends on whether the animal is a companion
animal or a livestock animal bred for human consumption.
The safety
documentation requirements for adjuvants used in human vaccines are, for
obvious reasons, higher. When used in preventive medicine the vaccine is
administered to healthy persons and in many cases, as part of vaccination
programs for children. Here adverse reactions to the adjuvant are not
acceptable.
With therapeutic
vaccines, however, a compromise is not unrealistic. Were therapeutic vaccines
against serious human diseases (e.g., HIV/AIDS or cancer) or therapeutic
vaccines against viral infections (e.g., HTLV-I or hepatitis C) to be developed
that required the help of strong adjuvants to be effective, less strict levels
of acceptance for the adjuvant side effects may be acceptable.
It would be a
question of balancing the profile of vaccination side effects against the
general prognosis for the disease if untreated or treated by other therapeutic
regimens, many of which themselves are not without side effects.
MECHANISMS
BEHIND ADJUVANT SIDE EFFECTS:-
The majority of adjutants
produce some effects at the injection site, the most frequent being an infl ammatory response. For the better
tolerated adjuvants, used in practical vaccination, by far the majority of
cases lead to transient and negligible symptoms only: mild pain, transient
swellings, and so on. However, among more than 100 different compounds, described
as adjuvants in the literature, the vast majority have been shown to be too reactogenic to be used in human as
well as veterinary applications. Such adjuvant active substances may
nevertheless be valuable tools for studying the immune system as such,
including side effects from excessive stimulation of the immune system.
The mechanisms
behind adjuvant side effects, as described below, comprise both observations
from the investigation of such highly reactogenic adjuvants (or cytokines) and
observations from signifi cant overdosing of classical adjuvants.
Local reactions
seen after the use of such adjuvants may range from local pain and erythemas to
granulomas, cysts, abscesses, and ulcers, particularly if overdosing
the adjuvant beyond the acceptable dose ranges.Adverse systemic reactions due
to adjuvant- or cytokine-induced stimulation of the immune system, including
pyrogenicity , flu like symptoms, and auto immune disorders, are known from
experimental immunology, but are, of course, disqualifying for use of the
adjuvant in practical vaccination. A number of observations of side effects
seen after vaccination with adjuvanted vaccines must, however, be attributed to
the vaccine preservatives (e.g., thiomersal, β-propriolactone, or formaldehyde)
or, as mentioned, to bacterial toxins from the antigen preparation.
Local Reactions: Effect of the Injection Modus
Vaccinations may be
given subcutaneously or intramuscularly. Other administration routes,
such as the intraperitoneal route
known from experimental immunology, are not used in practical parenteral
vaccination. Oral vaccination of humans
has been practiced against poliovirus since the 1960s, but this vaccine is not
adjuvanted. Quillaja saponin has
been used as an adjuvant for oral experimental vaccines and is accepted as a
food additive in Europe under code E999 due to low oral
toxicity. Hence, the potential of using Q.
saponin as an adjuvant for oral
immunizations is yet to be explored. Nasal immunization may have a future
in practical vaccination but is still at the developmental stage.
The injection modus
is not without importance in relation to local reactogenicity.
When immunizing by
the subcutaneous route the vaccine inoculum is introduced into a compartment
with numerous sensory neurons (in contrast to the intramuscular compartment).
The introduction of a local inflammatory response here may more easily give
rise to irritation, itching reactions, and local pain. Also, a transient
swelling, as a consequence of the inflammatory focus formed, may be palpable
more easily through the skin. After immunizing by the intramuscular route, even
a lot of similar size swelling may be less easily visible and palpable, as it
is located in deeper-lying tissue. Some
adjuvants (e.g., Q.
saponin) which show acceptable safety profiles when administered
intramuscularly or subcutaneously in rodents, may cause chemical peritonitis
and induce fibrous adherences in the body cavity when injected intraperitoneally.
Local Reactions: The Inflammatory Focus
Mineral adjuvants (aluminum- and calcium-based adjuvants)
should, along with water-in-oil emulsions, (Freund’s-type emulsion adjuvants)
be regarded as depot-forming or repository adjuvants. With these adjuvants the formation of a temporary
inflammatory focus attracting immunocompetent
cells shortly after injection must, more or less, be expected .Upon injection, phagocytic cells and APCs
are attracted to the site to phagocytize and clear the inoculum.
The local reaction
may be negligible if the inoculum is dispersed rapidly from the injection site.
However, if the inoculum resides for a prolonged period of time at the
injection site (as is the case with repository adjuvants) then in situ accumulation
of phagocytic and immunocompetent cells may in some cases manifest itself as an
inflammatory focus accompanied by transient swelling, local irritation, and
redness. There are observations of
aluminum-adsorbed vaccines giving lead to more local reactions than unadsorbed
vaccines with plain toxoid this could in part be explained by the plain toxoid
vaccine being dispersed from the injection site before a local reaction was
established.
Any visible or
palpable reaction at the injection site is in principle non grata, as it
hinders the obtaining of a hypothetical and nonreactogenic “ideal adjuvant.”
However, it is important to realize that the mechanisms described are part of a
normally functioning immune system. Hence, it may not be achievable to use
repository adjuvants without temporarily also inducing an infl ammatory focus
around the inoculum.
Attempts have been
made in recent years to link the presence of a local infl ammatory focus in the
myofascii [the condition is referred to as macrophagic myofasciitis (MMF)]
after intramuscular injections of aluminumadjuvanted vaccines to such
conditions as myalgia and muscle fatigue, but also to neurological disorders
with no obvious etiological relation to the vaccination Such correlations are,
however, associated with statistical problems. There is very high vaccination
coverage in Western countries. Hence, it is expected statistically that
patients as suffering from a wide range of etiologically unrelated diseases
would all have been vaccinated with aluminum-containing vaccines at some point
in their medical history. Another problem is that adequate statistical control
groups of non vaccinated persons may be hard to find in the same population.
In a recently published controlled study in primates by Verdier and coworkers in France, it was not possible to detect any histological changes after injection of aluminum-adjuvanted vaccine besides the local inflammatory focus itself, and they found no abnormal clinical signs associated to it.
Conclusion:
- Adjuvants are essential for the development of new and
improved vaccines.
- The development of successful vaccine adjuvants has been a
constant balancing act between safety and immunogenicity, delivery and
immunostimulation.
- The design and selection of new adjuvants will have to face
some major hurdles like:-
– Understanding of the mechanisms of adjuvanticity,
–
Development of appropriate delivery systems.