Revolutionizing Medication Production: Capsule Filling Machines in the Pharmaceutical Industry
Introduction
The pharmaceutical industry is at the forefront of healthcare innovation, and one critical aspect of this sector is the development and production of various dosage forms. Capsules, in particular, have become a popular choice for drug delivery due to their versatility, ease of administration, and precise dosage control. Behind the scenes, capsule filling machines play a pivotal role in pharmaceutical manufacturing, ensuring the efficient and accurate encapsulation of medications. In this comprehensive guide, we will explore the world of capsule filling machines, their historical evolution, types, components, working principles, filling processes, quality control measures, advantages, limitations, recent innovations, and their future prospects.
The Crucial Role of Capsule Filling Machines
Capsules, as a dosage form, have gained immense popularity in the pharmaceutical industry for several reasons:
Precision Dosage: Capsules allow for the precise dosing of medications, ensuring that patients receive the correct amount of active pharmaceutical ingredient (API).
Versatility: They can accommodate various drug formulations, including powders, pellets, beads, and even liquids, making them suitable for a wide range of pharmaceutical applications.
Ease of Administration: Capsules are easy to swallow, making them a preferred choice for patients, including those who may have difficulty taking tablets or liquids.
Protection of Ingredients: Capsules provide a protective shell that shields the enclosed medication from external factors, such as moisture and light, which can degrade the drug.
Evolution of Capsule Dosage Forms
The use of capsules in medicine dates back centuries. Historically, they were crafted manually, a time-consuming and labor-intensive process. Over time, technological advancements led to the development of capsule filling machines, revolutionizing pharmaceutical manufacturing. Today, these machines play a critical role in ensuring the efficiency, accuracy, and safety of capsule production.
Understanding Capsule Filling Machines
Definition and Functionality
How Capsule Filling Machines Work:
Capsule filling machines, as the name suggests, are devices designed to fill empty capsule shells with precise quantities of pharmaceutical formulations. They operate based on specific mechanisms, ensuring accurate dosage and consistent results.
Types of Capsules: Hard vs. Soft:
Capsules come in two primary forms—hard gelatin capsules and soft gelatin capsules. Hard capsules consist of two separate pieces, while soft capsules are a single, sealed unit. The choice between these two types depends on the medication’s characteristics and the desired dosage form.
Historical Overview
Early Encapsulation Methods:
Before the advent of modern capsule filling machines, the encapsulation process was labor-intensive and required skilled artisans. The earliest capsules were crafted manually using materials like animal intestines.
Advancements in Capsule Filling Technology:
The late 19th and 20th centuries saw significant developments in capsule manufacturing. Innovations such as semi-automatic machines, followed by fully automatic capsule fillers, transformed the industry. These advancements allowed for increased production capacity and improved dosage accuracy.
Types of Capsule Filling Machines
Capsule filling machines come in various types, each suited to specific production requirements and pharmaceutical applications.
Automatic Capsule Fillers
Features and Capabilities:
Automatic capsule fillers are high-speed machines capable of filling thousands of capsules per hour with precision and efficiency. They are often used in large-scale pharmaceutical manufacturing.
High-Speed vs. Low-Speed Machines:
Automatic capsule fillers come in high-speed and low-speed variants, catering to different production needs. High-speed machines are ideal for large batches, while low-speed machines are suitable for smaller runs and research purposes.
Semi-Automatic Capsule Fillers
Operational Characteristics:
Semi-automatic capsule fillers strike a balance between automation and operator control. They require manual loading of empty capsules but automate the filling and closing processes.
Applications in Pharmaceutical Production:
These machines find applications in pharmaceutical development, where flexibility and small batch sizes are essential. They allow researchers to experiment with various formulations.
Manual Capsule Fillers
Utility in Research and Development:
Manual capsule fillers are primarily used in research and development settings. They provide researchers with a cost-effective and hands-on approach to capsule filling, allowing for customization and flexibility.
Customization and Flexibility:
Researchers can easily adjust fill weights, experiment with different formulations, and produce small quantities of capsules for testing.
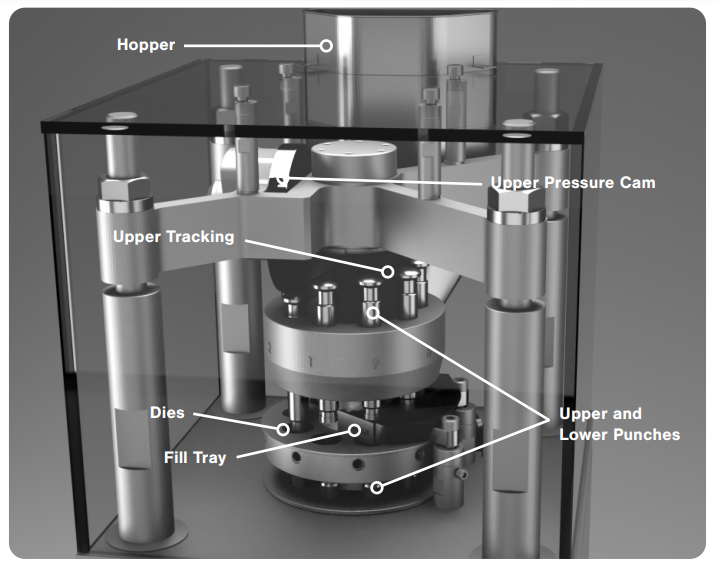
Components and Working Principles of Capsule Filling Machine
Capsule filling machines are complex devices comprising various components, each with a specific role in the encapsulation process.
Dosage Disc and Tamping Pin
Ensuring Precision and Consistency:
The dosage disc is a critical component responsible for metering the precise quantity of powder or formulation to be filled into each capsule. The tamping pin then compacts the material, ensuring uniform dosage.
Role in Dosage Formulation:
The design and adjustment of the dosage disc and tamping pin play a crucial role in achieving dosage accuracy and consistency.
Powder Hopper and Agitator
Managing Powder Flow:
The powder hopper stores the pharmaceutical formulation to be encapsulated. An agitator within the hopper ensures consistent powder flow to the filling stations.
Preventing Material Segregation:
Effective powder management prevents material segregation, ensuring that the capsule contents are homogeneous.
Capsule Magazine and Feeder
Feeding Capsules into the Filling Stations:
The capsule magazine stores empty capsules, while the feeder delivers them to the filling stations as needed.
Avoiding Cross-Contamination:
Capsule magazines and feeders are designed to prevent cross-contamination between different formulations or batches.
Filling Stations and Dosing Mechanisms
Accurate Powder Dispensing:
The filling stations are where the actual filling of capsules takes place. Dosing mechanisms dispense precise amounts of the pharmaceutical formulation into the capsules.
Adjusting Fill Weights:
The versatility of capsule filling machines allows for easy adjustment of fill weights to meet specific dosage requirements.
Closing and Ejection Mechanisms
Sealing the Capsules:
Once filled, the capsules are sealed using a closing mechanism, ensuring they remain intact until administration. Different machines employ various closing techniques, including mechanical, tamping, or ultrasonic sealing.
Removing Filled Capsules:
Ejection mechanisms safely remove the filled capsules from the machine for further processing or packaging.
Control Systems and Monitoring
Automation and Data Logging:
Modern capsule filling machines are equipped with advanced control systems that automate various functions, such as monitoring fill weights, detecting and rejecting defective capsules, and logging production data.
Quality Assurance in Real-Time:
These systems contribute to quality assurance by providing real-time data on machine performance and product quality.
Capsule Filling Processes
Capsule filling machines can handle a variety of materials and formulations, including powders, pellets, beads, and even liquids.
Powder Filling
Techniques for Precise Dosage Filling:
Powder filling requires precise techniques to ensure uniform distribution within the capsule. Techniques like dosator, tamping pin, and vacuum filling are commonly employed.
Powder Flow Control:
Proper control of powder flow is crucial to prevent bridging or rat-holing issues during the filling process.
Pellet and Bead Filling
Encapsulation of Particulate Materials:
Capsules are ideal for encapsulating pellets or beads, allowing for controlled release formulations and multiparticulate drug delivery systems.
Applications in Controlled Release Formulations:
This method is particularly useful for medications that require extended-release profiles or taste masking.
Liquid Filling
Challenges and Solutions:
Filling capsules with liquids presents unique challenges, such as maintaining precise fill volumes and preventing leakage. Machines designed for liquid filling employ specialized mechanisms to address these challenges.
Expanding Capsule Applications:
Liquid-filled capsules are gaining popularity for delivering a wide range of pharmaceutical products, including suspensions, solutions, and oils.
Quality Control and Assurance
Ensuring the quality and safety of pharmaceutical products is paramount in the industry. Capsule filling machines are subject to rigorous quality control measures.
In-Process Monitoring
Ensuring Consistency:
Capsule filling machines incorporate in-process monitoring systems to detect variations in fill weights or capsule integrity. Any discrepancies trigger corrective actions, ensuring consistent dosages.
Reject and Reconciliation Procedures:
Machines equipped with reject mechanisms discard capsules that do not meet quality standards. Reconciliation procedures ensure accurate accounting of all capsules produced.
Cleaning and Maintenance
Preventing Cross-Contamination:
Thorough cleaning and maintenance protocols are in place to prevent cross-contamination between different products or batches. Cleaning validation is a critical aspect of pharmaceutical manufacturing.
Extending Machine Lifespan:
Regular maintenance routines not only ensure hygiene but also extend the lifespan of capsule filling machines, optimizing their efficiency.
Regulatory Compliance
GMP Requirements:
Capsule filling processes are governed by Good Manufacturing Practices (GMP) regulations. Compliance with these standards is essential to ensure product safety and quality.
Validation and Documentation:
Pharmaceutical companies must validate their capsule filling processes and maintain comprehensive documentation to demonstrate compliance with regulatory requirements.
Advantages and Limitations
Capsule filling machines offer numerous advantages but also come with specific limitations and challenges.
Advantages of Capsule Filling Machines
Precision and Dosage Accuracy:
Capsule filling machines ensure precise dosage, reducing the risk of dosage errors in pharmaceutical products.
Versatility and Flexibility:
They can accommodate various formulations, enabling the production of a wide range of medications.
Cost-Efficiency in Production:
High-speed automatic capsule fillers streamline production, reducing labor costs and enhancing efficiency.
Limitations and Challenges
High Initial Investment:
The purchase of capsule filling machines represents a significant upfront cost, which can be a barrier for smaller pharmaceutical companies.
Operator Training and Skill:
Effective operation and maintenance of capsule filling machines require skilled personnel who are trained in GMP and machine-specific procedures.
Specific Material Requirements:
Certain formulations may pose challenges, such as powder flow issues or the need for specialized capsule shells.
Innovations and Trends
The pharmaceutical industry is continually evolving, and capsule filling machines are no exception. Recent innovations are shaping the future of pharmaceutical manufacturing.
Automation and Industry 4.0 Integration
Robotics and AI in Capsule Filling:
Automation is advancing with the integration of robotics and artificial intelligence, enhancing production efficiency and reducing the need for human intervention.
Data-Driven Production Optimization:
Industry 4.0 concepts are being applied to capsule filling, enabling real-time data analysis for process optimization and quality control.
Sustainability Initiatives
Green Capsule Manufacturing:
Pharmaceutical companies are increasingly adopting sustainable practices in capsule production, including using eco-friendly materials and reducing energy consumption.
Reducing Waste in Production:
Efforts are underway to minimize waste generation during the capsule filling process, contributing to environmental conservation.
Personalized Medicine and Customization
Tailoring Dosage Forms for Patients:
Advancements in capsule filling technology allow for the customization of medication dosages to meet individual patient needs.
3D Printing of Capsules:
Emerging technologies like 3D printing are being explored for the on-demand production of personalized capsules.
Future Prospects and Challenges
As we look ahead, capsule filling machines are poised to play an even more significant role in pharmaceutical manufacturing. However, they also face certain challenges and considerations.
The Future of Capsule Filling
Emerging Technologies and Materials:
Advancements in materials science and technology will lead to the development of more efficient and adaptable capsule filling machines.
Meeting Global Healthcare Needs:
As healthcare demands grow, capsule filling machines will need to keep pace with the increasing production requirements.
Challenges on the Horizon
Regulatory Changes and Compliance:
The pharmaceutical industry is subject to evolving regulations, and capsule filling machines must continually adapt to meet compliance standards.
Competition and Market Dynamics:
Pharmaceutical manufacturers will face increasing competition and market dynamics that will require flexibility and efficiency in production.
Conclusion
Capsule filling machines are unsung heroes in the pharmaceutical industry, ensuring that medications are accurately dosed, efficiently produced, and safely delivered to patients. From their historical roots to the cutting-edge innovations of today, these machines have played a pivotal role in the evolution of pharmaceutical manufacturing. As the industry continues to advance, capsule filling technology will remain at the forefront, meeting the challenges and demands of an ever-changing healthcare landscape.
References
References that are used for the article on “Capsule Filling Machines in the Pharmaceutical Industry”:
FDA Guidance for Industry: Powder Blends and Finished Dosage Units – Stratified In-Process Dosage Unit Sampling and Assessment.
United States Pharmacopeia (USP) Chapter <711> Dissolution.
Capsules: Hard Shelled, 2020, Encyclopedia of Pharmaceutical Technology, Volume 3, pp. 565-579. DOI: 10.1081/E-EPT120039781
Lachman, L., Lieberman, H. A., & Kanig, J. L. (2012). The theory and practice of industrial pharmacy. Lippincott Williams & Wilkins.
Fiegel, J., Fu, J., Hanes, J., & Polymeric nanoparticles for oral drug delivery, Nanomedicine, Volume 3, Issue 1, 2008, Pages 125-138, ISSN 1743-5889, DOI
Vold, I. M. N., Škalko-Basnet, N., & Basnet, P. (2013). Analytical methods for the characterization of liposomes and exosomes. Therapeutic delivery, 4(9), 1117-1142.
United States Pharmacopeia (USP) Chapter <1217> Sterile Product Packaging – Integrity Evaluation. Link
Quality by Design for ANDAs: An Example for Immediate-Release Dosage Forms.
Please ensure to format these references according to the citation style (e.g., APA, MLA) you intend to use in your article.
This comprehensive guide provides an in-depth exploration of capsule filling machines in the pharmaceutical industry. From their historical development to the latest innovations, readers gain insights into the critical role these machines play in ensuring precise and efficient medication production. As pharmaceutical manufacturing evolves, capsule filling machines continue to be indispensable tools for delivering quality healthcare products to patients worldwide.
For more detailed guides on Pharmaceutical equipment pls refer this