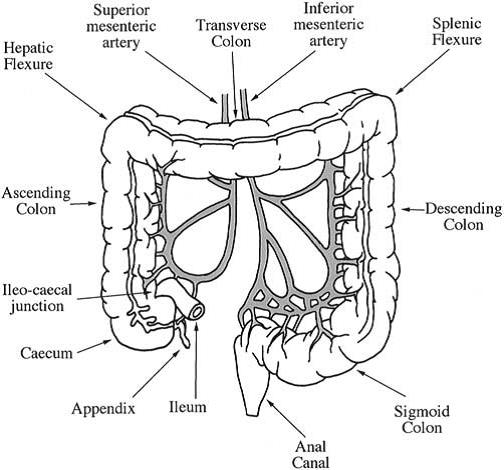
Pharmacology Study Notes: Colon : COLON SPECIFIC DRUG DELIVERY SYSTEM
FACTORS TO BE CONSIDERED IN THE DESIGN OF COLON SPECIFIC DRUG DELIVERY SYSTEM
To reach the colon and to be able to specifically deliver and absorb the drug there, the dosage forms must be formulated taking into account the obstacles of the gastrointestinal tract. The various strategies developed to achieve this goal have used the specific characteristics of this organ, i.e. transit time, pH, microflora, enzymes, disease and the colonic environment. Nevertheless, these parameters can vary from one individual to the next and also according to the pathological condition and diet.
Physiological Factors
Gastrointestinal transit
Gastrointestinal transit time is important for nearly all orally targeting delivery systems. The drug delivery systems first enter in to stomach and small intestine via mouth and then reach colon. In fasted state, the motility proceeds through four phases occurring in stomach and small intestine that span over a period of 2-3 h. Phase I is a quiescent period of 40-60 min, Phase II consists of intermittent contractions for a period of 40-60 min. Phase III is a period of intense contractions sweeping material out of the stomach and down the small intestine followed by Phase IV with contractions dissipating. The feeding state affects the normal pattern by irregular contractile activity.
It has been well documented that gastric emptying varies with different types of dosage forms. Examples of gastric residence times of single-unit tablets are given in Table 1 (Abrahamsson, 1993). It has been generally accepted that liquid emptying follows a monoexponential process and digestible solids empty in a linear fashion with time.
Small intestinal transit
Normally, transit times through the small intestine generally found to be 3-4 h. Liquids, small solids (beads, small tablets), and larger capsule-sized units moved essentially at the same rates and the transit is unaffected by food status (Davis, 1986). In a more recent study concerning dosing in relation to the timing of food intake, found that although SIT is relatively independent of food and dosage form, it was actually shortened significantly if the dose is given 30 min before food intake. This can have adverse impact on the in vivo performance of the dosage forms.
Colonic Transit
In the stomach and small intestine, food residue and endogenous secretions are exposed to an essentially sterile environment through which their transit can be measured by hours. On entering the large intestine, dosage forms encounter a rich bacterial flora and transit through the large intestine can be as long as several days. It was reported that overall mean transit time is 36 h with a range of 1 to 72 h and that the transit of liquids and small solids is equal (Phillips., 1993). Thus, absorption from colon may be incomplete and erratic depending on the dose and physicochemical properties of a particular drug. In general, absorption of an insoluble drug with high dose or a drug with limited permeability is unfavorable in this region because of the limited volume of fluid available for dissolution and the significantly reduced surface area.
Pharmacology Study Notes Colon COLON SPECIFIC DRUG DELIVERY SYSTEM pdf Pharmacology Study Notes Colon COLON SPECIFIC DRUG DELIVERY SYSTEM 
Table 1. Gastrointestinal transit times for felodipine CR hydrophilic matrix
| section | Gastric emptying (h) | Small intestine transit (h) | Colon arrival (h) | |||
| Fasting | Fed | Fasting | Fed | Fasting | Fed | |
| Mean | 0.6 | 3.2 | 4.7 | 5.1 | 5.3 | 8.3 |
| Range | 0.1-1.1 | 1.9-4.8 | 3.9-5.9 | 2.2-7.7 | 4.0-7.0 | 6.0-11.0 |
| P | <0.001 | >0.05 | >0.01 | |||
pH in the Colon
The pH gradient in the GIT is not in an increased order and is subjected to both inter- and intra-subject variations. In stomach the pH is 1.5 – 2.0 and 2 – 6 in fasted and fed conditions, respectively. The acidic pH is responsible for the degradation of various pH sensitive drugs and enteric coating may prevent it. In small intestine, the pH increases slightly from 6.6 – 7.5. On entry into the colon, the pH dropped to 6.4 in right colon. The pH of mid colon was found to be 6.6 and in the left colon, 7.0 (Evans et al., 1988).
Colonic pH has been shown reduced in disease state. The mean pH in a group of 7 patients with untreated ulcerative colitis was 4.7 whereas in 5 patients receiving treatment it was 5.5 (Raimundo et al., 1992).
Colonic microflora
The human colon is a dynamic and ecologically diverse environment, containing over 400 distinct species of bacteria with a population of 1011 to 1012 CFU/mL (Cummings et al., 1991), with Bacteroides, Bifidobacterium, Eubacterium, Lactobacillus, etc greatly outnumbering other species. For example, it was reported that Bacteroides, Bifidobacterium and Eubacterium could constitute as much as over 60% of the total cultivable flora (Salyers, 1984). These bacteria produce a wide spectrum of enzymes that, being reductive and hydrolytic in nature, are actively involved in many processes in the colon, such as carbohydrate and protein fermentation, bile acid and steroid transformation, metabolism of xenobiotic substances, as well as the activation and destruction of potential mutagenic metabolites. Nitroreductase, azoreductase, N-oxide and sulfoxide reductase are the most extensively investigated reductive enzymes, while glucosidase and glucuronidase are the most extensively studied hydrolytic enzymes. The primary source of nutrition for these anaerobic bacteria is carbohydrates such as non-starch polysaccharides (i.e., dietary fibers) from the intestinal chime. It is well established that non-starch polysaccharides are fermented during transit through the colon and the breakdown in the stomach and small intestine is negligible. Enzymes responsible for the degradation of polysaccharides include α-L-arabinofuranosidase, β-D-fucosidase, β-D-
galactosidase, β-Dglucosidase, β-xylosidase, with the last three enzymes being the most active (Englyst et al., 1987). Additionally, the composition of colonic bacteria and corresponding enzymes can be influenced by many factors, including age, diet, diseases, medication such as antibiotics, and geographic regions (Mueller et al., 2006). A unique feature of colon microflora is that the growth and activity of certain specific species, most notably bifidobacteria and lactobacilli, can be selectively stimulated by nondigestible oligosaccharides which are known as prebiotics. Similar bacteriological data were observed in the rats fed with indigestible oligosaccharides where the caecal bifidobacteria population was higher than in the controls (Campbell et al., 1997).
Volume of the ascending colon
Up to 1,500 g of liquids and undigested materials (dietary fibers, resistant starch, partially degraded polysaccharides proteins, mucins, exfoliated epithelial cells, etc.) enters colon per day, which act as the substrates for microflora fermentation. Water together with the products of the fermentation and other nutrients was efficiently absorbed in the colon, condensing the contents into feces through the transit in the colon for eventual defecation. Therefore, it is very likely that the ascending colon contains the largest quantity of liquid. It would be expected that the low water–high gas environment of the transverse colon limits dissolution of materials. The moisture content of caecal contents is believed to be about 86% (Cummings and Macfarlane, 1991). The volume of the ascending colon was measured in healthy subjects using a single photon emission computed tomography (SPECT) by acquiring the imaging of the ascending colon filled with 99Tcm-labelled Amberlite pellets, and was found to be 170±40 ml (Badley., 1993). If the moisture content in the ascending colon is approximately comparable to that of caecal contents, the quantity of fluid in the ascending colon should be 146±34 ml.
Pharmaceutics notes B Pharmacy M pharmacy Study Material
Disease and the Colonic Environment
General intestinal diseases such as inflammatory bowel disease, Crohn’s disease, constipation and diarrhea may affect the release and absorption of colon specific drug delivery systems. All the specific approaches so far mentioned rely on the concept that enzymes produced by colonic microflora provide the trigger for specific delivery of fermentable coatings, anti-inflammatory azobond drugs, and other prodrugs to the cecum. Carrette and co-workers (1995) demonstrated that in patients with active Crohn’s disease, the metabolic activity of digestive flora (assessed on the activity of fecal glycosidases) was decreased. Azoreductase activity in feces of 14 patients with active Crohn’s disease was 20% of that of healthy subjects and similarly, beta-D-glucosidase and beta-D-glucuronidase activities in fecal homogenates incubated under anaerobic conditions were also decreased in patients. These data probably reflect large-bowel hypermotility and the associated diarrhea, leading to lower bacterial mass in the colon and might contribute to the therapeutic failure of targeting mechanisms in active ileocolic and colic Crohn’s disease.

 Click here to download
Click here to download