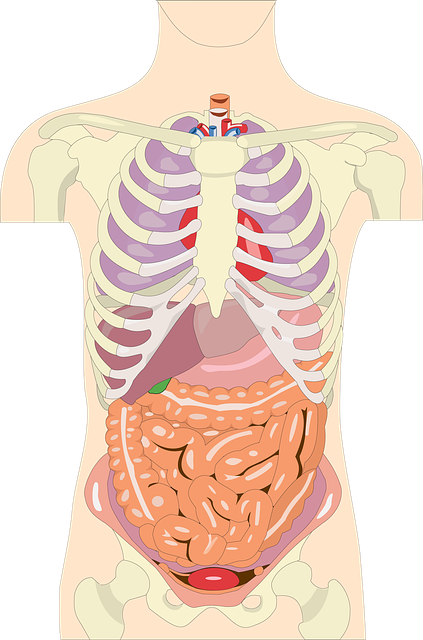
M Pharmacy Project : APPROACHES TO COLON-SPECIFIC DRUG DELIVERY
APPROACHES TO COLON-SPECIFIC DRUG DELIVERY
In recent years, a large number of solid formulations targeting the lower parts of the GI tract, especially the colon, have been reported. These formulations may be broadly divided into four types, which are
- pH-dependent system designed to release a drug in response to change in pH,
- Time controlled ( or Time-dependent) system designed to release a drug after a predetermined time,
- Microbially-controlled system making use of the abundant enterobacteria in the colon,
- Enzyme-based systems – Prodrug, and
- Pressure-dependent system making use of luminal pressure of the colon.
Among these, first three are most widespread formulation technologies being developed for pharmaceutical market.
pH-DEPENDENT SYSTEMS
Solid formulations for colonic delivery that are based on pH-dependent drug release mechanism are similar to conventional enteric-coated formulations but they differ in target site for delivery and therefore type of enteric polymers. In contrast to conventional enteric-coated formulations, colonic formulations are designed to deliver drugs to the distal (terminal) ileum and colon, and utilize enteric polymers that have relatively higher threshold pH for dissolution (Dew et al., 1982; Tuleu et al., 2001). Most commonly used polymers (Table 2) are derivatives of acrylic acid and cellulose. These polymers have ability to withstand an environment ranging from low pH (~1.2) to neutral pH (~7.5) for several hours. Apparently, it is highly desirable for pH-dependent colonic formulations to maintain their physical and chemical integrity during passage through the stomach and small intestine and reach the large intestine where the coat should disintegrate to release the drug locally. It should be however noted that GI fluids might pass through the coat while the dosage form transits through the small intestine. This could lead to premature drug release in the upper parts of GI tract and as a result loss of therapeutic efficacy may occur. One approach to overcome this problem is to apply
higher coating levels of enteric polymers; however, this also allows influx of GI fluids through the coat, and the thicker coats often rupture under the influence of contractile activity in the stomach. In general, the amount of coating required depends upon the solubility characteristics (solubility, dose/solubility ratio) of the drug, desired release profile and surface area of the formulation, and composition of the coating solution/dispersion.
Widely used polymers are methacrylic resins (Eudragits), which are available in water soluble and water-insoluble forms. Eudragit L and S are copolymers of methacrylic acid and methyl methacrylate. To overcome the problem of premature drug release, a copolymer of methacrylic acid, methyl methacrylate and ethyl acrylate (Eudragit® FS), which dissolves at a slower rate and at a higher threshold pH (7–7.5), has been developed recently. A series of in vitro dissolution studies with this polymer have highlighted clear benefits over the Eudragit® S polymer for colonic targeting (Rudolph et al., 2001).
![M Pharmacy Parmaceutics Project APPROACHES TO COLON-SPECIFIC DRUG DELIVERY [Ceutics]](https://pharmawiki.in/wp-content/uploads/2017/10/M-Pharmacy-Parmaceutics-Project-APPROACHES-TO-COLON-SPECIFIC-DRUG-DELIVERY-Ceutics.png) M Pharmacy Project APPROACHES TO COLON-SPECIFIC DRUG DELIVERY pdf M Pharmacy Project APPROACHES TO COLON-SPECIFIC DRUG DELIVERY
M Pharmacy Project APPROACHES TO COLON-SPECIFIC DRUG DELIVERY pdf M Pharmacy Project APPROACHES TO COLON-SPECIFIC DRUG DELIVERY
Khan et al., (1999) prepared lactose-based placebo tablets and coated using various combinations of two methacrylic acid polymers, Eudragit® L100-55 and Eudragit® S100 by spraying from aqueous systems. The Eudragit® L100-55 and Eudragit® S100 combinations studied were 1:0, 4:1, 3:2, 1:1, 2:3, 1:4, 1:5 and 0:1. The coated tablets were tested in vitro for their suitability for pH dependent colon targeted oral drug delivery. The same coating formulations were then applied on tablets containing mesalazine as a model drug and evaluated for in vitro dissolution rates under various conditions. The disintegration data obtained for the placebo tablets demonstrate that disintegration rate of the studied tablets is depends on the polymer combinations used to coat the tablets, pH of the disintegration media and the coating level of the tablets. Dissolution studies performed on the mesalazine tablets further confirmed that the release profiles of the drug could be manipulated by changing the Eudragit® L100-55 and Eudragit® S100 ratios within the pH range of 5.5 to 7.0 in which the individual polymers are soluble respectively, and a coating formulation consisting of a combination of the two copolymers can overcome the issue of high GI pH variability among individuals. The results also demonstrated that a combination of Eudragit® L100-55 and Eudragit® S100
could be successfully used from aqueous system to coat tablets for colon targeted drug delivery and the formulation can be adjusted to deliver drug at any other desirable site of the intestinal region of the GIT on the basis of pH variability.
Colon targeted drug delivery systems based on methacrylic resins has described for insulin (Touitou and Rubinstein., 1986), prednisolone (Thomos., 1985), quinolones (Van Saene et al., 1986), salsalazine (Riley et al., 1987), cyclosporine (Kim et al., 2001), beclomethasone dipropionate (Levine et al., 1987) and naproxane (Hardy et al., 1987). pH-sensitive delivery systems are commercially available for mesalazine (5-aminosalicylic acid) (Asacol® and Salofalk®) and budesonide (Budenofalk® and Entocort®) for the treatment of ulcerative colitis and Crohn’s disease, respectively.
Table 2. Threshold pH of commonly used polymers
| Polymer
|
Threshold pH |
| Eudragit® L100
Eudragit® S100
Eudragit® L 30D
Eudragit® FS 30D
Eudragit® L100-55
PVAP
HPMCP
HPMCP 50
HPMCP 55
CAP |
6.0
7.0
5.6
6.8
5.5
5.0
4.5-4.8
5.2
5.4
5.0 |
PVAP = Polyvinyl acetate phthalate; HPMCP = Hydroxypropylmethylcellulose phthalate; CAP= Cellulose acetate phthalate
TIME-CONTROLLED (OR TIME-DEPENDENT) SYSTEMS
Time-controlled systems are useful for synchronous delivery of a drug either at pre-selected times such that patient receives the drug when needed or at a pre-selected site of the GI tract. These systems are therefore particularly useful in the therapy of diseases, which depend on circadian rhythms. Time-controlled formulations for colonic delivery are also delayed-release formulations in which the delay in delivery of the drug is time-based. In these systems, it has been suggested that colonic targeting can be achieved by incorporating a lag time into the formulation equivalent to the mouth to colon transit time (Chourasia and Jain, 2003). Ideally, formulations are designed such that the site of delivery (i.e. colon) is not affected by the individual differences in the gastric emptying time, pH of the stomach and small intestine or presence of anaerobic bacteria in the colon. A nominal lag time of 5 h is usually considered sufficient, since small intestinal transit has been considered relatively constant at 3 to 4 h. In principle, time-controlled systems rely on this consistent small intestinal transit time. The drug release from these systems therefore occurs after a predetermined lag phase, which is precisely programmed by selecting a suitable combination of controlled-release mechanisms.
Available technologies based on the time controlled systems are
- Codes system – comprises a series of polymers that are combined to protect the drug core until the formulation arrives in the colon.
- Colon-Targeted Delivery System – uses lag time to achieve colon delivery. The system is comprised of three parts: an outer enteric coat, an inner semipermeable polymer membrane, and a central core comprising swelling excipients and an active component.
- Oros-CT – is a technology developed by Alza Corporation and consists of an enteric coating, a semipermeable membrane, a layer to delay drug release, and a core consisting of two compartments.
- Time Clock – delivery device developed by Pozzi and colleagues is a pulsed delivery system based on a coated solid dosage form.
The first formulation introduced based on this principle was Pulsincap® (MacNeil et al., 1990). It is similar in appearance to hard gelatin capsule; the main body is made water insoluble (exposing the body to formaldehyde vapour which may be produced by the addition of trioxymethylene tablets or potassium permanganate to formalin or any other method). The contents are contained within a body by a hydrogel plug, which is covered by a water-soluble cap. The whole unit is coated with an enteric polymer to avoid the problem of variable gastric emptying. When the capsule enters the small intestine the enteric coating dissolves and the hydrogels plug starts to swell, the amount of hydrogel is such adjusted that it pops out only after the stipulated period of time to release the contents. The viability of such a system in human volunteers has been confirmed on the basis of evaluation studies (Binns et al., 1994).
In a study by Gazzaniga et al., (1995) a novel oral time based drug release system was developed, containing core coated with three polymeric layers. The outer layer dissolves at pH > 5, then the intermediate swellable layer, made of an enteric material. The system provides the expected delayed release pattern, as also indicated by the preliminary in vivo studies on rats. Several other drug delivery systems have developed that rely upon the relatively constant transit time of small intestine (Gupta et al., 2001; Fukui et al., 2000).
Another formulation approach to achieve time-dependent delivery to the colon is osmotically controlled system (Figure 2). Theeuwes et al., (1990) described a delayed-release osmotic delivery device that can be used for localized treatment of colonic diseases or for achieving systemic absorption of drugs that are otherwise unattainable. The delivery system, commonly referred as push-pull OROS system, comprises as many 5 push-pull units encapsulated within a hard gelatin capsule. Each push-pull unit is a bilayered laminated structure containing an osmotic push layer and a drug layer, both surrounded by a semipermeable layer (approx. 0.076 mm thickness). In principle, the semipermeable membrane is permeable to the inward entry of water or aqueous GI fluids and is impermeable to the outward exit of the drug. An orifice is drilled through thesemipermeable membrane next to the drug layer. The outside surface of the semipermeable membrane is then coated by Eudragit® S-100 (approx. 0.076 mm thickness) to delay the drug release from the device during its transit through the stomach. Upon arrival in the small intestine, the coating dissolves at pH >7. As a result, water enters the unit causing the osmotic push compartment to swell, forcing the drug out of the orifice into the colon. The drug release kinetics is precisely controlled by the rate of influx of water through the semipermeable membrane. For treating the ulcerative colitis, each push pull unit is designed with a 3-4 h post gatric delay to prevent drug delivery in the small intestine.
Figure 2: Cross section of the OROS-CT colon targeted drug delivery system
![APPROACHES TO COLON-SPECIFIC DRUG DELIVERY [Ceutics notes]](https://pharmawiki.in/wp-content/uploads/2017/10/APPROACHES-TO-COLON-SPECIFIC-DRUG-DELIVERY-Ceutics-notes.png)
MICROBIALLY-CONTROLLED SYSTEMS
These systems are based on the exploitation of the specific enzymatic activity of the microflora (enterobacteria) present in the colon. The colonic bacteria are predominately anaerobic in nature and secrete enzymes (azoreductases, β-glucuronidase, β-xylosidase, dextranases, esterases, nitroreductase, etc.) that are capable of metabolizing substrates such as carbohydrates and proteins that escape the digestion in the upper GI tract.
Polysaccharides offer an alternative substrate for the bacterial enzymes present in the colon. A number of naturally occurring polysaccharides are stable in the upper intestine yet susceptible to hydrolytic degradation in the lower intestine (Sinha et al., 2003; Vandamme et al., 2002). Most polysaccharides can be chemically modified to optimize specific properties, such as the ability to form impermeable films (Hovgaard et al., 1996). Table 3 lists a number of polysaccharide-based oral delivery systems for targeted release in the lower intestine (David R. Friend, 2005). Some of these systems have been tested in humans.
Pectin is a non-starch linear polysaccharide composed mainly of α-(1→4)-linked D-galacturonic acid groups with some 1→2 linked L-rhamnose groups. Pectin, like many other polysaccharides, is stable in the stomach and small intestine but susceptible to enzymatic degradation in the large intestine (Rubinstein et al., 1993). Calcium (Rubinstein and Glixokabir, 1995) and zinc salts (Cooke, 1967) of pectin are preferred for lower intestinal delivery since they have lower water solubility and hence better dissolution delaying properties than sodium pectinate or pectic acid. To further delay release of drugs, compression coating around a core containing drug has also been studied (Rubinstein and Radai., 1995). Improved targeted delivery to the lower intestine using pectin and other naturally occurring polysaccharides is accomplished by coating tablet or multiparticulate formulations with traditional enteric polymers. This formulation approach was tested in a human study with normal volunteers using gamma scintigraphy.
The formulations were composed of enteric-coated calcium pectinate matrix tablets prepared with and without guar gum as a binder. The tablets were found to reach the colon in most cases intact and there they disintegrated.
Another approach used to limit drug dissolution in the upper intestine involves mixed films. Mixed films are composed of polysaccharides coformulated with water-insoluble polymers such as ethylcellulose or chitosan (partially deacetylated chitin) and gel forming polymers such as hydroxypropylmethylcellulose (HPMC). These mixed films were used to prepare coatings for tablets to deliver drugs into the colon. In vitro dissolution testing of the coated tablets using a pectinolytic enzyme preparation showed that drug release was accelerated by action of this enzyme preparation compared with dissolution medium free of the enzyme (MacLeod et al., 1999).
Another polysaccharide examined for its ability to delay release of drugs in the GI tract is guar gum (GG). GG is a galactomannan material composed of linear chains of (1→4)-β-D-mannopyranosyl units with α-D-galactopyrannosyl units linked by (1→6). The colon contains enzymes (galactomannanases) capable of degrading GG (Gibson et al., 1990) to short chain fatty acids. Both matrix tablets and compression coated tablets have been administered in humans.
Tablets composed primarily of GG and the drug dexamethasone were dosed orally in humans and their transit and disintegration followed using gamma scintigraphy (Kenyon et al, 1997). Some drug was released from the tablets prior to colonic arrival but the majority of drug was released in the large intestine and release was generally correlated with tablet disintegration. A similar study resulted in the same results although no drug was used in the formulations (Krishnaiah et al., 1998). The results generated in these two studies suggested that a compression coating approach could improve targeted release (Krishnaiah et al., 1999).
The use of GG as a compression coating to delay release of a drug (rather than a gamma emitting substance) has been studied recently. Following in vitro studies (Krishnaiah et al., 2002a) a GG-based colon targeted oral delivery system for the drug 5-fluorouracil was tested in a group of 12 healthy volunteers (Krishnaiah et al., 2003a). The results from this study are consistent with delivery of 5-fluorouracil to the large intestine:
tmax increased from 0.6±0.01 h (immediate release tablets) to 7.6±0.1 h. There was no drug detected in the plasma until approximately 5 h had elapsed. In most instances, assuming normal transit patterns, the tablets are located in the colon at this time. Similar data have been obtained with several other drugs (mebendazole, metronidazole, celecoxib, and tinidazole) (Krishnaiah et al, 2003b, 2002b 2002c, 2002d).
Xanthan gum is a high molecular weight extracellular polysaccharide, produced on commercial scale by the viscous fermentation of gram negative bacterium Xanthomonas campesteris . The molecule consists of a backbone identical to that of cellulose, with side chains attached to alternate glucose residues. It is a hydrophilic polymer, which until recently had been limited for use in thickening, suspending and emulsifying water based systems. It appears to be gaining appreciation for fabrication of matrices, as it not only retards drug release, but also provides time- independent release kinetics with added advantages of biocompatibility and inertness. Release of soluble drugs was mainly through diffusion, whereas sparingly soluble or insoluble drugs were released via erosion. It is also recommended for use in both acidic and alkaline systems.
Polysaccharide-based formulations represent a relatively simple formulation approach that can be scaled-up and prepared in a reproducible and inexpensive manner. If there are no chemical modifications to the polysaccharide (i.e., they meet compendial monographs such as USP/NF), most can be used in products without additional safety testing.
Table 3: Polysaccharide-based materials used to deliver drugs to the lower Intestine
| Polysaccharide |
Dosage forms
investigated |
References |
| Pectin
Calcium salt
Methoxylated
Derivatives
Mixed films
of pectin |
Matrices, compression
coated tablets, Compression coating
Film coating for tablets
and beads |
Rubinstein et al., 1993; 1995
Ashford et al., 1994
Wakerly et al., 1996; MacLeod et al., 1999 |
| Chitosan
Chitosan
Chitosan derivatives |
Coated capsules and
Microspheres
Matrices |
Tozaki et al., 1997
Aiedeh et al., 1999 |
| Guar gum
Guar gum
Guar gum –
derivatives |
Matrix tablets,
compression coated
tablets
Coatings or matrix
Tablets |
Krishnaiah et al., 1998a; 1999; 2002a; 2003a
Rubinstein et al., 1995; Gliko-Kabir et al., 2000 |
| Chondroitin sulfate
Cross-linked
chondroitin |
Matrix tablets |
Rubinstein et al., 1992a, |
| Alginates
Calcium salt |
Swellable beads |
Shun et al., 1992 |
| Inulin
Mixed films |
Tablet and bead coatings |
Vervoort et al., 1996 |
| Dextran
Diisocyanate
cross-linked dextran |
Hydrogels |
Brbndsted et al, 1995; Chiu et al.,1999 |
ENZYME-BASED SYSTEMS – PRODRUG
A successful prodrug-based delivery system is one in which the promoiety (i.e, inactive portion of the prodrug) minimizes absorption until the active is released (usually by enzymatic action) near the target site. Thus, the promoiety is used to increase the hydrophilicity of the parent drug, increase molecular size, or both, thus minimizing absorption of the drug prior to reaching the target site (Sinha and Kumria., 2001).
This principle has been exploited commercially to deliver 5-aminosalicylic acid to the colon by way of a prodrug carrier. The prodrug sulphasalazine consists of two separate moieties, sulphapyridine and 5-aminosalicylic acid, linked by an azo-bond. The prodrug passes through the upper gut intact, but, once in the colon, the azo-bond is cleaved by the host bacteria, liberating the carrier molecule sulphapyridine and the pharmacologically active agent 5-aminosalicylic acid (Travis et al., 1994). This concept has led to the development of novel azo-bond-based polymers (azo-polymers) for the purpose of obtaining universal carrier systems. However, issues with regard to the safety and toxicity of these synthetic polymers have yet to be addressed.
Cyclodextrins (CyDs) have been proposed as inert carriers for targeting in the GIT. Since CyDs are poorly absorbed from the GIT due to their size and hydrophilicity and degraded in the large intestine, it is possible to use them as carriers for delivery of drugs in the lower intestine. α, β, and γ-CyD-drug conjugates of prednisolone were prepared and tested as potential colon-specific prodrugs (Yano et al, 2001a, 2001b; 2002).
It has been proved through a study in healthy human volunteers that β-CyDs are meagerly digested in small intestine but are completely degraded by the microflora of the colon. The anti-inflammatory effect and systemic side effect of the prednisolone succinate/alpha-cyclodextrin ester conjugate after oral administration were studied using IBD model rats. The systemic side effect of the conjugate was much lower than that of prednisolone alone when administered orally. The lower side effect of the conjugate was attributable to passage of the conjugate through the stomach and small intestine without significant degradation or absorption, followed by the degradation of the conjugate site-specifically in the large intestine (Yano et al., 2002).
A related approach based on polysaccharides involves the use of dextrans. Like CyDs, they are relatively stable in the upper intestine but subject to enzymatic hydrolysis in the lower intestine by dextranases produced by gut microflora. A simple approach to linking a drug to dextran involves attaching carboxyl acid groups on the drug to hydroxyl groups on the polymer. In the absence of a carboxylic acid group on the drug, a spacer molecule such as succinic or glutaric acid can be used (Harboe et al., 1988).
PRESSURE-DEPENDENT SYSTEM
Another approach to controlling the site (and potentially the rate) of drug release in the GIT is using the pressure. Due to the reabsorption of water from the large intestine, the viscosity of the luminal contents increases (Digenis and Sandefer., 1991). As a result, intestinal pressures increase due to peristalsis in the distal intestine providing a potential means to trigger release of a drug from a formulation susceptible to pressure changes. Such a formulation approach, called pressure- controlled colon delivery capsule (PCDC) system has been examined in both animals and humans (Takada et al., 1995).
Formulations susceptible to changes in pressure are prepared from capsule-shaped suppositories coated with ethylcellulose. The materials used in preparation of the suppositories are polyethylene glycols (PEGs). They are selected so that they melt at body temperature. The system behaves as a balloon once the PEG liquefies. In the upper intestine, there is sufficient fluidity to maintain the integrity of balloon and no drug release occurs. In the large intestine however, pressures induced by peristalsis directly affect the EC balloon leading to rupture and subsequent drug release.
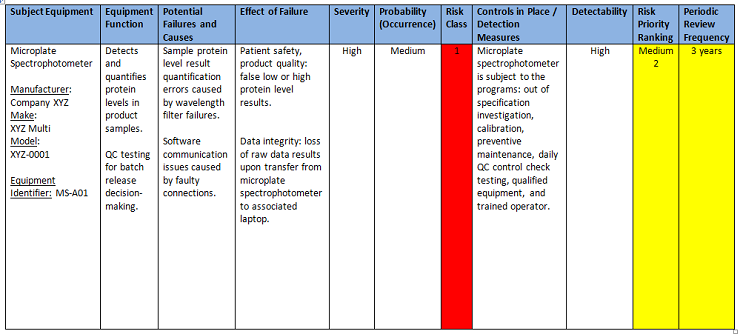





![[PPT PDF] Pharmaceutical Water System Validation - IDENTIFICATION OF MICROORGANISMS](https://pharmawiki.in/wp-content/uploads/2017/11/PPT-PDF-Pharmaceutical-Water-System-Validation-IDENTIFICATION-OF-MICROORGANISMS.jpg)
![Pharmaceutical Water Systems Pharmaceutical Water Storage & Distribution Systems [PDF PPT]](https://pharmawiki.in/wp-content/uploads/2017/11/Pharmaceutical-Water-Systems-Pharmaceutical-Water-Storage-Distribution-Systems-PDF-PPT.jpg)
![M Pharmacy Parmaceutics Project APPROACHES TO COLON-SPECIFIC DRUG DELIVERY [Ceutics]](https://pharmawiki.in/wp-content/uploads/2017/10/M-Pharmacy-Parmaceutics-Project-APPROACHES-TO-COLON-SPECIFIC-DRUG-DELIVERY-Ceutics.png)
![APPROACHES TO COLON-SPECIFIC DRUG DELIVERY [Ceutics notes]](https://pharmawiki.in/wp-content/uploads/2017/10/APPROACHES-TO-COLON-SPECIFIC-DRUG-DELIVERY-Ceutics-notes.png)