Hello readers, Todays article deals with vaccine adjuvants. We focus on the Characteristics of ideal adjuvant, Mechanisms of adjuvant action, Types of adjuvants and learn them in detail. The majority of vaccine antigens presently under investigation represent highly purified recombinant molecules or sub-units of pathogens and, hence, they lack many features of the original pathogens, including the inherent immunostimulatory property. Thus, the development of safe and potent immunologic adjuvants and delivery systems that can enhance and direct vaccine-specific immunity is needed.
Since the earliest attempts to raise significant immune responses against nonliving agents, investigators have tried to identify useful additives that can be combined with antigens to enhance immune responses. Such immuneenhancing additives are known as adjuvants. Virtually all adjuvant systems developed to date have focused on one of two mechanisms: specific immune activation or the delivery–depot effect. Although many adjuvant systems have been developed and tested in preclinical models, few have actually proved useful for human vaccines. The primary limitations for the use of new adjuvant systems with human vaccines revolve around safety issues. Whereas the toxicity of adjuvants has been reduced systematically through research and development efforts over the last 80 years, the safety barriers presented by regulatory and liability issues have continued to increase. Adjuvants to be used with prophylactic vaccines in normal, healthy populations need to have virtually pristine safety profiles. The fact that most vaccines today are given to infants or children heightens the safety concerns of vaccine adjuvants.
- Characteristics of
ideal adjuvant
- Mechanisms of
adjuvant action
- Types of adjuvants
- Aluminum-containing
adjuvants
- Mf59:a oil-in-water
emulsion
- Microorganism –
derived adjuvants
- Biodegradable
nanoparticles
- Poly(lactide-co-glycolide)
microparticles
- Nucleic acid –
based adjuvants
- Polyphosphazenes
as vaccine adjuvants
- Archeosomes as
vaccine adjuvants
Characteristics of an Ideal Adjuvant
• Safety of adjuvant must be assured, including freedom from immediate and long term side effects.
• Adjuvant must be chemically and biologically defined.
• Ensure batch to batch variations to assure consistency.
• The adjuvant combined with antigens should elicit a more robust protective immune response than do such antigens without adjuvant.
• Efficacy should be achieved with lower concentrations of antigen than that required for aqueous vaccine.
• The adjuvant vaccine should be stable on the shelf for at least 2 years to be commercially and clinically useful.
• The adjuvant should be biodegradable and easily removed from the body after its adjuvant effect is exhausted.
• The adjuvant should be inexpensive.
• The adjuvant would be effective in infants and young children, ideally at birth, and elicit a more persistent response of high quality (high affinity antibodies or desired tvpe of IgG isotype).
Examples and Types of Modern Vaccine Adjuvants:-
Classes
of Modern Vaccine Adjuvants:-
- Aluminium
and Calcium salts
e.g., aluminium hydroxide; aluminum
phosphate and
Calcium phosphate.
- Oil emulsions and
surfactant based formulations,
e.g., MF59
(microfluidised detergent stabilised oil-in-water emulsion),
QS21 (purified
saponin),
AS02 [SBAS2]
(oil-in-water emulsion + MPL + QS-21),
Montanide ISA-51 and
ISA-720(stabilised water-in-oil emulsion).
Deoxycholic
acid/alum complex
Dimethyl dioctadecyl
ammonium bromide
Avridine
Nonionic
block copolymers – Pluronics
e.g., virosomes (unilamellar
liposomal vehicles incorporating influenza haemagglutinin)
AS04 ([SBAS4] Al salt with MPL)
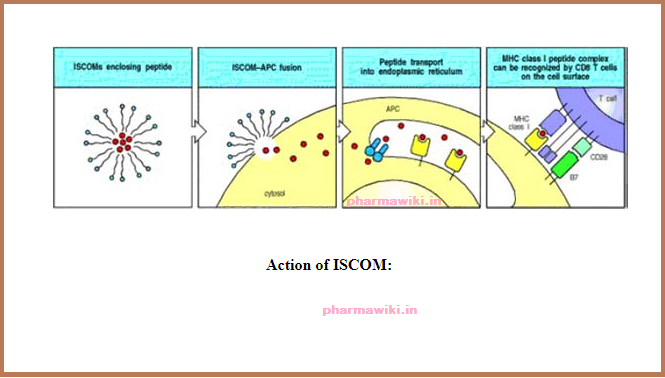
ISCOMS (structured
complex of saponins and lipids),
polylactide
co-glycolide (PLG) microparticles
- Microbial
derivatives (natural and synthetic)
Complete Freund’s adjuvant (killed M. tuberculosis);
DETOX (Cell wall skeleton
of M.PhleI + MPL);
Trehalose dimycolate
BCG
Muramyl dipeptides and tripeptides
Monophosphoryl lipid A (MPL)
Gamma inulin and
algammulin
Betglucan (algal glucan.Pleuran)
Neuraminidase-galactose
oxidase
Klebsiella
Pneumoniae glycoprotein
Bordetella pertussis
Corynebacterium Parvuni
AGP [RC-529] (synthetic acylated
monosaccharide)
DC_Chol (lipoidal immunostimulators able to
self organiseinto liposomes),
OM-174 (lipid A derivative),
CpG motifs (synthetic oligonucleotides
containing immunostimulatory CpGmotifs),
Modified LT and CT (genetically modified
bacterial toxins to provide non-toxic adjuvant
effects).
- Endogenous
human immunomodulators
Cytokines – Granulocyte-macrophage colony
stimulating factor,
INF-a, INF-g, IL-1, IL-2, IL-7, IL-12
hGM-CSF
or hIL-12 (cytokines that can be administered either as protein or plasmid
encoded),
Immudaptin
(C3d tandem array)
1. Dextran
2. Double stranded polynucleotides
3. Acetylated polymannose
4. Sulfolipopolysaccharide
Polymethyl methacrylate (PMMA)
Carbopol
Vitamins
– Vitamin A, D, E
Hormones – Human growth hormone,
Dehydroepiandrosterone
Imidazo-quinolines – Imiquimod
Glycolipid bay R1005
Stearyl
tyrosine
7-allyl-8-oxoguanosine
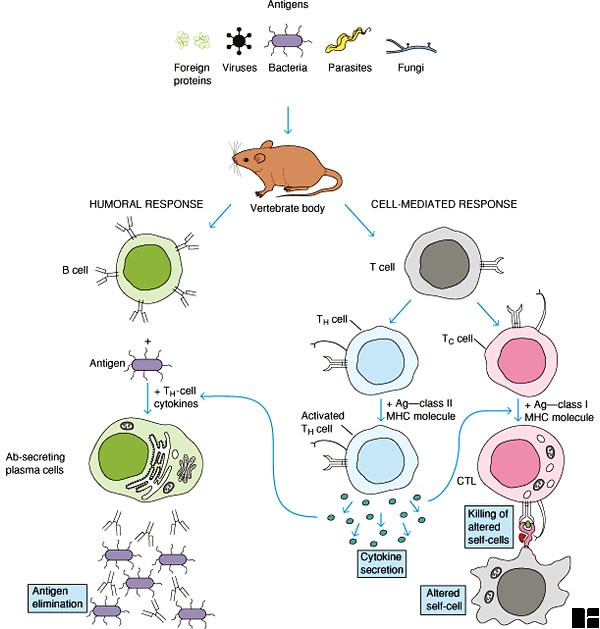
Mechanisms of adjuvant action:
Adjuvants may exert their immune-enhancing effects according to the following immune-functional activities:
• Adjuvants help in the translocation of antigens to the lymph nodes where they can be recognized by T cells.
• Adjuvants provide physical protection to antigens which grants the antigen a prolonged delivery.
• Adjuvants help to increase the capacity to cause local reactions at the site of injection, inducing greater release of danger signals by chemokine releasing cells such as helper T cells and mast cells.
• Adjuvants are believed to increase the innate immune response to antigen by interacting with Toll-like receptors (TLRs )on accessory cells.
• Introduce Ag into the appropriate in vivo microenvironment.
• Retain and release Ag slowly from the site of deposition.
• Recruit and activate Ag-presenting cells and lymphoid cells.
• Activate complement and induce synthesis, secretion, and binding of cytokines.
• Deliver T-cell epitopes to the MHC class I (cytoplasmic) pathway of Ag presenting cells for CD8-cytotoxic T-lymphocyte induction.
• Deliver T-cell epitopes to the MHC class II (Phagolysosome) pathway of Ag presenting cells for CD4-T-lymphocyte mediated responses and antibody induction.
• Activation of complement and stimulation of macrophages to induce retention and activation of lymphocytes and lymph nodes.
• Facilitate mucosal immunization by binding to M cells and promoting uptake of particles.