Pharmacology noted for B pharmacy and M Pharmacy students is readily available in our site www.pharmawiki.in/ [Doc PDF PPT ] Adenosine: Pharmacology notes B pharm M pharmacy Study Material is here below. Have a look at it and study it.
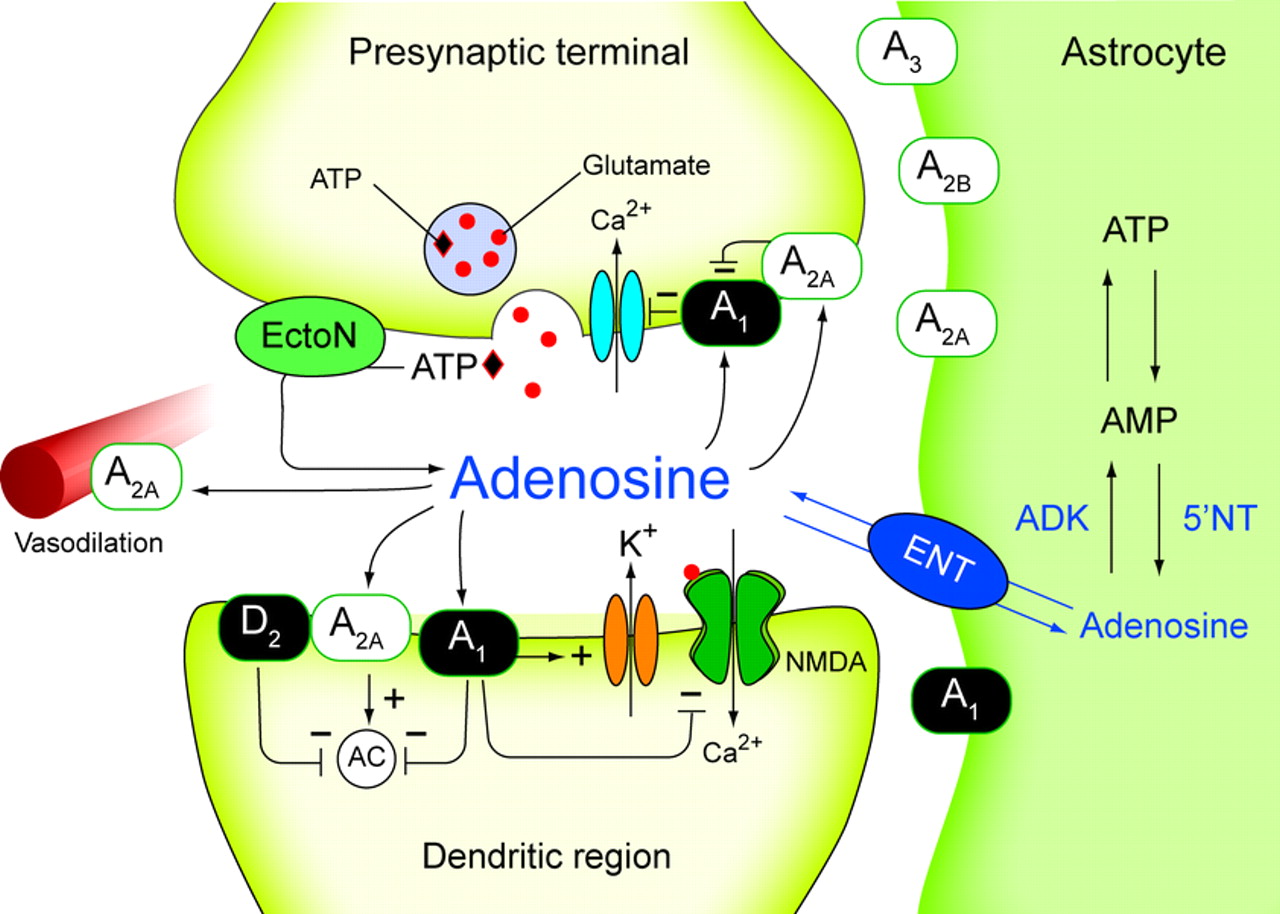
Adenosine is a purine nucleoside that regulates many physiological functions which includes respiratory regulation, neural function ,platelet aggregation, hormonal action , lymphocyte differentiation, vascular tone, negative chronotropic and dromotropic effect on heart , also mediates inhibition of neurotransmitter release and lipolysis . These physiological function have been largely revised.(1),(2)
These functions are mediated through different adenosine receptor. There are four subtypes of AR-A1,A2A-AR,A2B-AR,A3-AR each of these receptors has distinct tissue distribution and effector coupling. They belong to super family of G-protein coupled receptors (3).among these receptors A1,A3AR1 are closely related based on their sequence similarity while A2A,A2B AR also similarly related. A1 and A3 are primarly couple to G(subi) –family of G-protein.A2A and A2B are mostly coupled to GS like G- protein. Each of these receptors plays an essential role in responding to adenosine in central nervous system(4) ,regulating pain (5) . cerebral blood flow(6). Basal gangalia function (7) respiration (8) and sleep (9.) thus these receptors can be therapeutic targets for several diseases. Development of more selective agonists and antagonists for adenosine receptor subtype provide aclass of therapeutics for treatment of numerous human diseases such as apain (10). parkinsons disease (11) asthma(12) huntingtons disease(13).A search for new leads acting on specific adenosine acting on specific adenosine receptors may provide a key for novel therapeutics
Structure of A2A AR
A2A-AR subtype is linked to and G(S) and G(OLF) protein and up on activation the intracellular levels of Camp are increased . the expression A2A AR expression is higest in brain, .spleen,thymus,leucocyte and blood platelets and intermediate in heart lungs and blood vessel.(14)(15)..Crystal structure of A2A AR was determined in 2008,physiological functions A2A AR are regulation of sensori motors integration in basal ganglia., inhibition of platelet aggregation and polymorpho nuclear leucocytes, vasodilation protection against ischemic damage, stimuation of sensory nerve activity . (17) these wide range of functions implies their significant role in the body and use of chemical moieties to alter these function in disease state (may be agonists or antagonists).
A2A AR Adenosine antagonists:
A2A AR antagonist have their role in parkinsons disease, (18) keep regulations (19) controlling alcohol abuse (20) invivo receptor imaging (21) there can also be used an anti depressant drug. (22) A2 AR agonists can be a treatment for ischemic renal injure (23) paraoxysmal supro ventricular tachycardia. They can be used as vasodilators (24) antithrombic agent (25) antinflamatory (26) . they can also be used in treatment of asthma( 27), arthritis(28) sepsis (29) inflamatory bowel disease (30) and reduced skin pressure ulcer formation (26) and accelerator wound healing,(31)
In view of the role of A2A AR in these diseases afurther study in to the subject may reveal beneficial (facts) information for the treatment of such dieases. These receptors became agood targeting strategy to bring out novel therapeutics for effective treatment of dieases.
Click here to download Pharmacology notes for B pharmacy M pharmacy StudentsADENOSINE
[DOC] Adenosine. Pharmacology notes B pharm M pharmacy study material
[Webpage] Adenosine. Pharmacology notes B pharm M pharmacy study material
[PDF] Adenosine. Pharmacology notes B pharm M pharmacy study material
Google Drive Link for Adenosine. Pharmacology notes B pharm M pharmacy study material
Pharmacology study material ADENOSINE Article REFRENCES:
(1) K. A. Jacoboson, Z.G.Gao, Adenosine receptors as therapeutic targets Nal.Rev., Drug Discovery 5(2006) 247-264
(2)M.P.Abbracchio, G.BUrnstock, A.verkhasatsky, H. Zimmermann, purinergic signalling in nervous system an over view ,Trends Neurosci. 32 (2009) 19-29
(3)B.B. Fredholm, G.Arsian, L.Halldner, B.kull, G.Schutte, W.Wasserman, structure and function of adenosine receptors and their genes , Naunyn – Schmiedebergs Arch.pnaarmacol.362 (2006) 19-29
(4)(a) . T.V Dunwiddie, S.A .Masina Annu . Rev.Neurosci 24,31(2001)
(b). K.A.Jacobson, Z.G.Gao , Nat .Rev Drug Discover.5,247 (2006)
(5) J.sawynok,x.J.Liv,Drog Neurobio . 69,313 (2003)
(6) Y.Shietal, J.Cereb Blood Flow Method . 28,111 (2008)
(7) M.A.Schwarzchild , L.Agnati, K. Fure, J. Fichsn, M.M orelli , Trends Neurobiol 29. 647 (2006)
(8) S.Lahiri, CH.Nitchell.D.Reigade , A.Roy , N.s.chemiack , Respir. Physiol. Nenrobiol. 157 ,123 (2007)
(9) R.Basheer,R.E.Strecker,MM.Thatkar,R.W.M.Carley Prog. Neurobiol.73,379 (2009)
(10)J,Sawynok,X.J.Liu. Prpg.Neurobio.69,313
(11)A.H.Schapira.et.al, Nat.Rev.Drug.Discor.5,845 (2006)
(12)A. Brow, D.Spina, C.p.page, Br.J.Pharmacol.153,(suppli),5446(2008)
(13)D.Blum, R.Hourez, M.C.Galar, P.Popoli,S.N.Schiftmann, Lancet Neurol.2,366(2003)
(14)F.Meng, G.X.Xic, D.Chalmeri, C.Margan,S.J.Watson,Jr.,H.Akil,Cloning and expression of the A2a receptors from guinea pig brain Neuro chem 66(1996) 613-621
(15)R.A.Deter freud,M.Maccollin,J.Gusella,J.S.Flink Characterization and expression of the human A2a adenosine receptors gene, Neuro chem. 66(1996)362-368.
(16)Veli-Pekka Jaakola, Mark.T.Grifftin,Micheal, A.Hanson, Vadim cherezov , ellen y.t chien, J.Robert lane ,Adlioan, P.L.Jzerman, Raymond c.sterenes, the 2.6 Angstroun Crystal structure of a human A2a Adenosine receptor Bound to an Antagonist
(17)B.B.Fredholm, Adenosine, an endogenous distress signal, modulates tissue damage and repair not cell death and differentiation (2007) 14, 1315-1323
(18)Michael A .Schwarzschild, Luigi Agnati, Kjell Fuxe ,Jiang – fanchen and micaela morelli, Targetting Adenosine A2a receptors in parkinsons disease Trends in neurosciences Vol.29 No.11
(19) Satoh, S., Matsumura, H. & Hayaishi, O. Involvement of adenosine A2A receptor in sleep promotion. Eur. J. Pharmacol. 351, 155–162 (1998
(20) Yao, L. et al. dimers mediate synergy of dopamine D2 and adenosine A2 receptor-stimulated PKA signalling and regulate ethanol consumption. Cell 109, 733–743 (2002).
(21) Moresco, R. M. et al. In vivo imaging of adenosine A2A receptors in rat and primate brain using [11C]SCH442416. Eur. J. Nucl. Med. Mol. Imaging 32,405–413 (2005).
(22) El Yacoubi, M. et al. Absence of the adenosine A2A receptor or its chronic blockade decrease ethanol withdrawal-induced seizures in mice. Neuropharmacology 40, 424–432 (2001).
(23) Okusa, M. D. et al. A2A adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am.J. Physiol. Renal Physiol. 279, F809–F818 (2000).
(24) Fredholm, B. B., IJzerman, A. P., Jacobson, K. A., Klotz, K. N. & Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 53,527–552 (2001).
(25) Varani, K. et al. Dose and time effects of caffeine intake on human platelet adenosine A2A receptors:functional and biochemical aspects. Circulation 102,285–289 (2000)
(26) Peirce, S. M., Skalak, T. C., Rieger, J. M.,Macdonald, T. L. & Linden, J. Selective A2A adenosine receptor activation reduces skin pressure ulcer formation and inflammation. Am. J. Physiol. Heart Circ. Physiol. 281, H67–H74 (2001).
(27) Fozard, J. R., Ellis, K. M., Villela Dantas, M. F., Tigani, B. & Mazzoni, L. Effects of CGS 21680, a selective adenosine A2A receptor agonist, on allergic airways inflammation in the rat. Eur. J. Pharmacol. 438, 183–188 (2002).
(28) Montesinos, M. C. et al. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum. 48, 240–247 (2003
(29) Sullivan, G. W., Fang, G., Linden, J. & Scheld, W. M. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J. Infect. Dis. 189, 1897–1904 (2004).
(30) Odashima, M. et al. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 129, 26–33 (2005).
(31) Montesinos, M. C. et al. Wound healing is accelerated by agonists of adenosine A2 (Gs-linked) receptors. J. Exp. Med. 186, 1615–1620 (1997).






![[PDF PPT] Biotechnology Plant Design - Biotech Laboratory DESIGN CONSTRUCTION & VALIDATION OF GMP](https://pharmawiki.in/wp-content/uploads/2017/04/Biotechnology-Plant-Design-1.jpg)

![[PDF PPT] Biotechnology Plant Design - Biotech Laboratory DESIGN CONSTRUCTION & VALIDATION OF GMP](https://pharmawiki.in/wp-content/uploads/2017/04/Biotechnology-Plant-Design-1-300x209.jpg)