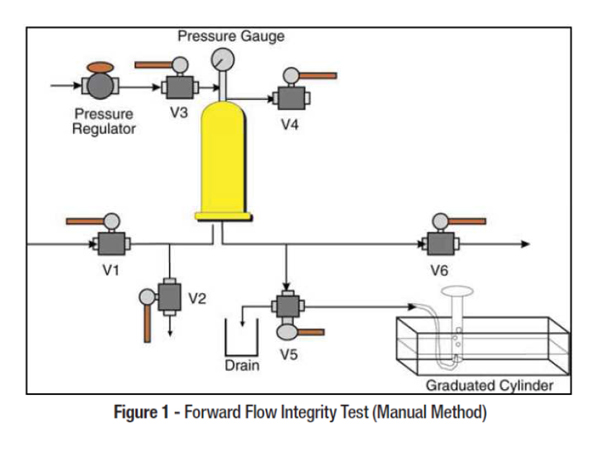
Water storage and distribution systems
Pharmaceutical Water Systems:: Water storage and distribution systems applies to WPU systems for PW, BHPW and BWFI. The water storage and distribution should work in conjunction with the purification plant to ensure delivery of water of consistent quality to the user points, and to ensure optimum operation of the water purification equipment.
General Principles of Water storage and distribution systems of Pharmaceutical Water Systems:
- The storage and distribution system should be considered as a key part of the whole system and should be designed to be fully integrated with the water purification components of the system.
- Once water has been purified using an appropriate method it can either be used directly or, more frequently, it will be fed into a storage vessel for subsequent distribution to points of use. The the requirements for storage and distribution systems and point of use fflPOU) is provided below.
- The storage and distribution system should be configured to prevent microbial proliferation and recontamination of the water fflPW, BHPW, BWFI) treatment. It should be subjected to a combination of online and offline monitoring to ensure that the appropriate water specification is maintained.
2 Materials that come into contact with systems for water for pharmaceutical use in Pharmaceutical Water Systems:
Here we deal with generation equipment for PW, BHPW and BWFI and the associated storage and distribution systems.
2.2 The materials that come into contact with WPU, including pipework, valves and fittings, seals, diaphragms and instruments, should be selected to satisfy the following objectives.
Compatibility.
The compatibility and suitability of the materials should encompass the full range of its working temperature and
potential chemicals that will come into contact with the system at rest, in operation and during sanitization.
Prevention of leaching.
All materials that come into contact with WPU should be non-leaching at the range of working and sanitization
temperatures of the system.
Corrosion resistance.
PW, BHPW and BWFI are highly corrosive. To prevent failure of the system and contamination of the water, the materials selected must be appropriate, the method of jointing must be carefully controlled and all fittings and components must be compatible with the pipework used. Appropriate sanitary specification plastics and stainless-steel materials are acceptable for WPU systems. When stainless steel is used it should be at least grade 316. In general 316L or a higher grade of stainless steel is used. The system should be passivated after initial installation or after significant modification. When accelerated passivation is undertaken the system should be thoroughly cleaned first and the passivation process should be undertaken in accordance with a clearly defined documented procedure.
Smooth internal Finish.
Once water has been purified it is susceptible to microbiological contamination and the system is subject to the formation of biofilms when cold storage and distribution are employed. Smooth internal surfaces help to avoid roughness and crevices within the WPU system. Crevices can be the source of contamination because of possible accumulation of microorganisms and formation of biofilms. Crevices are also frequently sites where corrosion can commence. The internal material finish should have an arithmetical average surface roughness of not greater than 0.8 micrometre fflRa). When stainless steel is used, mechanical and electro-polishing techniques may be employed. Electro-polishing improves the resistance of the stainless-steel material to surface corrosion.
Jointing.
The selected system materials should be easily joined by welding in a controlled manner. The control of the process should include, as a minimum, qualification of the operator, documentation of the welder set-up, work session test pieces logs of all welds and visual inspection of a defined proportion of welds, e.g. 100ft hand welds, 10ft automatic welds.
Documentation.
All system components should be fully documented and be supported by original or certified copies of material certificates.
Materials used for Pharmaceutical Water Systems:
Suitable materials that may be considered for sanitary elements of the system include 316L ffllow carbon) stainless steel, polypropylene, polyvinylidene-diFluoride and perFluoroalkoxy. The choice of material should take into account the intended sanitization method. Other materials such as unplasticized polyvinyl-chloride ffluPVC) may be used for treatment equipment designed for less pure water such as ion exchangers and softeners.
None of the materials that come into contact with WPU should contain chemicals that will be extracted by the water. Plastics should be non-toxic and should be compatible with all chemicals used. They should be manufactured from materials that should at least meet minimum food grade standards. Their chemical and biological characteristics should meet any relevant pharmacopoeia specifications or recommendations. Precautions should be taken to define operational limits for areas where water circulation is reduced and turbulent Flow cannot be achieved. Minimum Flow rate and change volumes should be defined.
3. System sanitization and bioburden control -Pharmaceutical Water Systems:
1 Water treatment equipment, storage and distribution systems used for BPW, BHPW and BWFI should be provided with features to control the proliferation of microbiological organisms during normal use, as well as techniques for sanitizing the system after intervention for maintenance or modification. The techniques employed should be considered during the design of the system and should take into account the interdependency between the materials and the sanitization techniques.
2 Systems that operate and are maintained at elevated temperatures ffle.g. > 65) are generally less susceptible to microbiological contamination than systems that are maintained at lower temperatures. When lower temperatures are required due to the water treatment processes employed or the temperature requirements for the water in use, special precautions should be taken to prevent the ingress and proliferation of microbiological contaminants fflsee section 6.4.3 for guidance).
4 Storage vessel requirements -Pharmaceutical Water Systems:
1 General
1 The water storage vessel used in a system serves a number of important functions. The design and size of the vessel should take into consideration the following.
2 Capacity
1 The capacity of the storage vessel should be determined on the basis of the following requirements:
It is necessary to provide a buffer capacity between the steady-state generation rate of the water-treatment equipment and the potentially variable simultaneous demand from user points.
The water-treatment equipment should be able to operate continuously for significant periods to avoid the equipment stress that occur when the equipment cycles on and off too frequently.
The capacity should be suffcient to provide short-term reserve capacity in the event of failure of the water-treatment equipment or inability to produce water due to a sanitization or regeneration cycle. When determining the size of such reserve capacity, consideration should be given to providing suffcient water to complete a process batch, work session, tank turnover by recirculation to minimize stagnation, or other logical period of demand.
3 Contamination control considerations -Pharmaceutical Water Systems:
The following should be taken into account for the efficient control of contamination:
) The headspace in the storage vessel is an area of risk where water droplets and air can come into contact at temperatures that encourage the proliferation of microbiological organisms. The use of spray-ball or distributor devices should be considered in these systems to wet the surfaces during normal operation, chemical and/or thermal sanitization.
) Nozzles within the storage vessels should be configured to avoid dead zones where microbiological contamination might be harboured.
) Vent filters are fitted to storage vessels to allow the internal level of liquid to Fluctuate. The filters should be bacteria-retentive, hydrophobic and should ideally be configured to allow in situ testing of integrity. Offline testing is also acceptable. The use of heated vent filters should be considered for continuous hot storage or systems using periodic heat sanitization to prevent condensation within the filter matrix that might lead to filter blockage and to microbial growth that could contaminate the storage vessels.
) Where pressure-relief valves and bursting discs are provided on storage vessels to protect them from under- and over-pressurization, these devices should be of a sanitary design. Bursting discs should be provided with external rupture indicators to ensure that loss of system integrity is detected.
Requirements for water distribution pipework -Pharmaceutical Water Systems:
General
The distribution of BPW, BHPW and BWFI should be accomplished using a continuously circulating pipework loop. Proliferation of contaminants within the storage tank and distribution loop should be controlled. Good justification for using a non-recirculating one-way system should be provided.
2 Filtration should not usually be used in distribution loops or at take off-user points to control biocontamination. Such filters are likely to conceal system contamination.
Temperature control and heat exchangers
Where heat exchangers are employed to heat or cool WPU within a system, precautions should be taken to prevent the heating or cooling utility from contaminating the water. The more secure types of heat exchangers of the double tube plate or double plate and frame or tube and shell configuration should be considered. Where these types are not used, an alternative approach whereby the utility is maintained and monitored at a lower pressure than the WPU may be considered. The latter approach is not usually adopted in BWFI systems.
Where heat exchangers are used they should be arranged in continually circulating loops or subloops of the system to avoid unacceptable static water in systems.
When the temperature is reduced for processing purposes the reduction should occur for the minimum necessary time. The cooling cycles and their duration should be proven satisfactory during the qualification of the system.
![Pharmaceutical Water Systems Pharmaceutical Water Storage & Distribution Systems [PDF PPT]](https://pharmawiki.in/wp-content/uploads/2017/11/Pharmaceutical-Water-Systems-Pharmaceutical-Water-Storage-Distribution-Systems-PDF-PPT.jpg)
3 Circulation pumps
Circulation pumps should be of a sanitary design with appropriate seals that prevent contamination of the system. Where stand-by pumps are provided, they should be configured or managed to avoid dead zones trapped within the system.
Consideration should be given to preventing contamination in systems where parallel pump systems are used, especially if there is stagnant water when one of the pumps is not being used.
4 Biocontamination control techniques
1 Water purification systems should be sanitized using chemical or thermal sanitization procedures as appropriate fflproduction and distribution). The procedure and conditions used fflsuch as times and temperatures) should be suitable.
2 The following control techniques may be used alone or more commonly in combination:
maintenance of continuous turbulent flow circulation within water distribution systems reduces the propensity for the formation of biofilms the system design should ensure the shortest possible length of pipework;
) for ambient temperature systems, pipework should be isolated from adjacent hot pipes;
) dead legs in the pipework should be minimized through appropriate design, and as a guide should not significantly exceed three times the branch diameter as measured from the ID pipe wall to center line of the point-of-use valve where significant stagnation potential exists;
) pressure gauges should be separated from the system by membranes;
) hygienic pattern diaphragm valves should be used;
) pipework for steam-sanitized systems should be sloped and fully drainable;
) the growth of microorganisms can be inhibited by:
– ultraviolet radiation sources in pipework;
– maintaining the system heated fflgreater than 65 °C);
– sanitizing the system periodically using hot water guidance temperature > 70’°C);
– sanitizing the system periodically using superheated hot water or clean steam;
– routine chemical sanitization using ozone or other suitable chemical agents. When chemical sanitization is used, it is essential to prove that the agent has been removed prior to using the water. Ozone can be effectively removed by using ultraviolet radiation.
Incoming searches for Pharmaceutical Water Systems:
Pharmaceutical Water Systems Pharmaceutical Water Storage & Distribution Systems PDF PPT
Pharmaceutical Water Systems Pharmaceutical Water Storage & Distribution Systems PPT
Pharmaceutical Water Systems
Purified Water Specification As Per Usp
Pharmaceutical Water System Design Operation And Validation Pdf
pharmaceutical water system design operation and validation
pharmaceutical water system ppt – What is Pharmaceutical water
purified water ff Water for Injection SOP as per usp
Pharmaceutical Water Systems: Storage ff Distribution Systems
pharmaceutical water system : Inspection of Pharmaceutical water systems
pharmaceutical water Production : Water purification systems
Pharmaceutical Water Systems: Types: Water quality specifications
Pharmaceutical Water System: principles for pharmaceutical water systems
Source: WHO






![Pharmaceutical Water Systems Pharmaceutical Water Storage & Distribution Systems [PDF PPT]](https://pharmawiki.in/wp-content/uploads/2017/11/Pharmaceutical-Water-Systems-Pharmaceutical-Water-Storage-Distribution-Systems-PDF-PPT.jpg)