Why students should do b pharmacy project at the end of the fourth year course?
Doing a B.Pharmacy project at the end of the fourth year course holds several significant advantages for students. Here are a few reasons why students should undertake a project as part of their B.Pharmacy curriculum:
Project offers Application of Knowledge:
A project provides an opportunity for students to apply the theoretical knowledge gained during their four-year course into practical scenarios. It helps bridge the gap between classroom learning and real-world application.
Skill Enhancement is core for B Pharm:
Engaging in a project enhances various skills such as research, critical thinking, problem-solving, data analysis, communication, and project management. These skills are highly valuable in both academic and professional settings.
Practical Experiential Learning through B pharm Projects:
Practical projects offer hands-on experience that textbooks and lectures cannot provide. Students get to face real challenges, make decisions, and learn from their successes and failures, which contributes to their overall development.
Industry Relevance Insight from Project you Choose:
B.Pharmacy projects often involve research or case studies related to the pharmaceutical industry. This experience gives students insights into the industry’s current trends, challenges, and innovations, making them better prepared for entering the job market.
Building Networking to grab Opportunities after course:
Students may collaborate with industry professionals, researchers, or faculty members during their project. This allows them to build a valuable network that can help them with career opportunities, references, and guidance.
Career Advancement Opportunity through right Project:
Undertaking a project can give students an edge in the job market. Employers often value candidates who have practical experience and a project demonstrates a student’s ability to independently manage and execute tasks. Hands on experience on something will give you an added advantage and gives you more points while getting hired.
Personal Development as a benefit :
Projects encourage self-discipline, time management, and self-motivation, which are vital skills for success in any field. Students also develop resilience as they tackle challenges throughout the project.
Project inculcate Problem Solving Skill in you:
Projects come in various forms, but they all have one thing in common – they require you to tackle real-world problems. Whether it’s formulating a new drug, conducting clinical trials, or addressing healthcare issues, these projects provide a unique opportunity for you to apply your problem-solving skills in a practical setting.
One of the key benefits of engaging in such projects is the improvement in your ability to address complex issues effectively. As you immerse yourself in these projects, you’ll encounter a wide range of challenges that require creative and analytical thinking. This will push you to think outside the box and find innovative solutions. This hands-on experience will not only deepen your knowledge but also enhance your problem-solving skills specific to that domain. Moreover, problem-solving projects provide an excellent opportunity for collaboration and teamwork.
Remember, developing problem-solving skills is not just about solving puzzles or riddles. It’s about gaining real-world experience and applying your knowledge to make a difference. So, embrace the opportunity to engage in problem-solving projects and watch your skills grow! In conclusion, participating in projects that involve problem solving is an excellent way to enhance your problem-solving skills
Projects often involve solving real-world problems related to drug formulation, clinical trials, or healthcare. These problem-solving experiences improve a student’s ability to address complex issues effectively.
Different Pharma Projects for Insights on Higher Education:
For students considering further studies like M.Pharmacy or research-based careers, a project provides a strong foundation in research methodologies and prepares them for more advanced academic pursuits.
Pharma Relevant Project Showcases as Achievements in Resume:
A successfully completed project can be added to a student’s portfolio, resume, or academic transcripts, highlighting their capabilities to potential employers or institutions.
In summary, a B.Pharmacy project serves as a culmination of a student’s academic journey, allowing them to apply their knowledge practically and develop essential skills for a successful career in the pharmaceutical field. It’s an opportunity to stand out, gain real-world experience, and contribute meaningfully to the industry.
Projects
List of B.Pharmacy & M. Pharmacy Projects to be done as a part of academic curriculum. Topics and ideas for project work of Pharmacy students are here today for you to help you a little bit in your academics to excel. When you are new to take up B.Pharm & M. Pharm project work we can say research work we http://pharmawiki.in are here today to help you choosing project title and topic. Topics for project in Pharmacology, Pharmaceutics, Pharmaceutical chemistry, pharmacognosy for B.Pharmacy & M. Pharmacy students in masters and bachelors final year. These Topics for project in Pharmacy for B.Pharmacy & M. Pharmacy will be of great help for the students who are going for the second year of master’s of pharmacy and also for the final year B.Pharmacy students.
How to Format References for Thesis & Research Paper Article
Reference formatting when you cite references in research article different publishers will follow the different guidelines according to their society rules. When writing a thesis for any piece of work you need to refer and your text material produced by other people. This procedure is called citing or quoting references. References need to be cited in two different places.
- At a point at which a document is referred to in the text of the work.
- In the list at the end of the work the bibliography other reference section
Let us discuss some general views on this.
Article Reference Formating
You put a superscript number after where you want to reference. I mean you can put a superscript number one after a statement.
You always need to put the reference after punctuation. For example if there is a comma and then the citation number.. If you have two citations for the same comment we put a comma between the two numbers separated by a space.
If you have more than two citations we put in the range of numbers separated by dash again after the punctuation point.
Author’s the journal title and then the author’s names the initials first separated by full stops and the last author is has the word and before him then you need to give the journal name appreciated appropriately and those abbreviations are available on the right side.
The year should be mentioned and insert a comma then give the volume in bold. Give issue number and then the first page number and then as a full stop.
Book Reference Formating
If its a Book reference you need to give the author’s names with and before the last author you’ve got the book title in italics. Then the publisher name. If there’s more than if this was more than the first edition we put in the Edition number. The publication site then the year of publication and all of that information is on the inside of a book cover.Book section reference and the page numbers at the end the rest of the format is the same so here we have the author who’s written a section in the book title we’ve got the book editors preceded by the words EDS heads had the publication year and strictly speaking this reference should also have the publication city and the publishers.
B.PHARMACY & M. PHARMACY PROJECTS: TOPICS FOR PROJECT WORK OF Pharmacognosy STUDENTS
Pharmacognosy B.PHARMACY & M. PHARMACY PROJECTS TOPICS
Effect of Natural Product Clove Bud Oil on Pathogenic Pseudomonas aeruginosa Virulence and Host Response
Phytochemical And Anti-Inflammatory Studies On The Hexane Extract Of The Stem Bark Of Steganotaenia Araliacea Hoschts (Apiaceae)
Phytochemical And Inhibition Studies Of Garcinia Kola Heckel (Guttiferae) Seed Extracts On Some Key Enzymes Involved With Diabetes
Phytochemical And Biological Studies On The Seeds Of Jatropha Curcas Linn. (Euphorbiaceae) –
Phytochemical And Anti Bacterial Studies On The Stem Bark Of Lannea Barteri. (Oliv.) Engl. (Anacardiaceae)
Pharmacognostic And Pro-Fertility Evaluations Of Dracaena Arborea (Willd) Linn. (Dracaenaceae) –
Pharmacognostic And Antimicrobial Studies On The Stem-Bark Of Ficus Kamerunensis Warb. (Moraceae)
Pharmacognostic And Antibacterial Studies Of The Leaf Extracts Of Swartzia Madagascariensis Desv (Fabaceae)
Pharmacognosy M. PHARMACY PROJECT TOPICS
Pharmacognostic And Antibacterial Studies Of Acacia Sieberiana Var Woodii (Fabaceae) Stem Bark –
Antimicrobial Property Of The Hexane Extract From The Pods Of Acacia Nilotica(L.) Del.
Phytochemical And Antimalarial Studies Of The Leaves Of Uvaria Chamae P.Beauv. (Annonaceae) –
Development And Validation Of Spectrophotometric Methods For The Determination Of Risperidone In Pure And Tablet Dosage Forms
Spectrophotometric Determination And Stability Studies Of Artemether In Artemether-Lumefantrine Suspensions Marketed In our Country, Nigeria
Phytochemical Studies And Effect Of Methanol Leaf Extract Of Leptadenia Hastata (Pers.) Decne (Asclepiadaceae) On Acetic Acid Induced Writhes In Mice And Venom Of Echis Ocellatus –
Phytochemical And Antimicrobial Studies On The Stem-Bark Of Commiphora Mollis (Oliv. ) Engl. (Burseracaea)
Phytochemical And Antimicrobial Studies Of Spermacoce Verticillata (Rubiaceae)
Comparative Evaluation Of The Ascorbic Acid Content Of Mineral Ascorbate And Ascorbic Acid Tablets Marketed In our Country
Pharmacognosy B.PHARMACY PROJECT TOPICS
Extraction, Formulation and Pharmacological evaluation of an Anti Microbial Cream Alexeyena Varghese
Pregnancy complications and role of life style modifications in a woman with Poly Cystic Ovary Syndrome (Pcos) Remya Reghu
Development and Validation of quantitative methods for the estimation of a drug in a dosage form Aneesh T. P.
Design, Synthesis and biological evaluation of Indole-3-Carbinol Sathianarayanan S.
Phytochemical, Pharmacological analysis and formulative study of aqueous extracts of dried galls of Quercus Infectoria Deepa T. Vasudevan
Study for assessment of knowledge , compliance and behavior of diabetes patients Meenu Vijayan
Preparation and evaluation of extended Release tablets Vidya Viswanad
Development and evaluationof Gastro- Retentive Floating Tablet(GRFT) of Rantidine Hydrochloride Swati Gupta
Role of Pharmacists in communication gap between Physician and Patient Leena Thomas
Study of dental problems in diabetic patients and their therapeutic management Naveen Kumar Panicker
Spasmolytic effect of (-) carvone on Isolated Vascular and Non-Vascular superfused smooth muscle preparations Mohamed Shabi
Human RBC Membrane stabilization study using Anti – inflammatory drug by In-vitro method Jipnomon Joseph
Evaluation of Antimicrobial Activity of Aqueous Leaf Extracts of Chrysophyllum cainito R. Aravind
Study on complication of Gestational Diabetes and its management in a tertiary care teaching hospital Roshni P. R.
Comparative study of different species of Tulasi for larvicidal activity Rahul R.
Formulation, evaluation and In-Vitro activity of Gel loaded with Quinex Moringa Litha Thomas
Anti oxidant Activity of chromene compounds and their microwave synthesis
B.PHARMACY & M. PHARMACY PROJECTS: TOPICS FOR PROJECT WORK OF Pharmaceutical Chemistry STUDENTS
Here are B.PHARMACY & M. PHARMACY PROJECTS: TOPICS FOR PROJECT WORK OF Pharmaceutical Chemistry STUDENTS. Medicinal Chemistry is a vast and pious branch in Pharmaceutical sciences. Many researchers and professors are into huge research. Drug Discovery is the trending research in Pharmaceutical chemistry branch of Medicinal Chemistry. Drug Design, synthesis and binding studies of different products along with traditional synthesis of products is current research era. Trying different and Innovative ways to drug discovery through receptors peptides Enzymes Harmon’s is one good thought for selecting some project in your B.PHARMACY & M. PHARMACY PROJECTS.
Pharmaceutical Chemistry Projects for M Pharmacy B Pharmacy
Design, synthesis and binding studies of water-soluble fluoride receptors
Developing an enzymatic toolbox to make complex modified peptides
Discovery of novel pharmaceuticals from marine and desert microorganisms
Synthesis and study of simplified vancomycin analogues as novel antibiotics
Organic crystals with large channels and nanopores
Innovative ways to disinfect surfaces using catalysis
Discovery of novel cancer immunotherapeutic agents
First-Principles Based Mechanochemistry of Pharmaceutical Active Ingredients
Molecular mechanism of action and inhibition of ATP synthase using biomolecular simulations
Carbon nanotubes in the boron neutron capture therapy of cancer
In silico modelling of protein dynamics in heart disease
Targeting the CXCR4-CB2 G-protein coupled receptor complex as a treatment for breast cancer
Shape Variant Nanoparticles for Pathogen Sensing
Magnetic hyperthemia for cancer treatment: synthesis, biofunctionalisation of nanoparticles for thermo-chemotherapy
Total Synthesis of the Dineolignan Ophiocerol and Derivatives
Synthesis of bioactive natural products and associated analogues
Chiral sulfoxide auxiliaries for the asymmetric synthesis of benzannulated spiroketals
Synthesis of unusual dispiro metabolites
Synthesis of danshenspiroketallactones and cryptoacetalides
Synthesis of Danshenspiroketallactone and Cryptoacetalide for the Treatment of Cardiovascular Disorders
Vaccine design for lectin targets
Organocatalytic assymetric synthesis of 6,5-benzannulated spiroketals
Synthetic and Computational Studies on Members of the Pyranonaphthoquinone Family of Anitbiotics
Design and synthesis of rat selective toxicants
Investigating the efficacy of novel antimicrobial mixes on microorganisms, surfaces and cells lines; an integrated study
Natural products as prophylaxis and treatment for gonococcal eye infections
Novel assays for screening drug – lipid membrane interactions
Organic/physical/computational chemistry – Improved methods for modelling reactivity and physical properties
Design and Synthesis of a Prospective Drug Candidate Against Diabetes
Synthesis of Mukanadin B and Analogues as Possible Neuroprotective Agents
B.PHARMACY PROJECTS TOPICS
- Anti Inflammatory Activity of Chalcones
- Design, Synthesis & Biological Evaluation of Antiprostrate Cancer Agents
- Studies on the constituents isolated from the Shizoines of Nervilia Aragoana GAUD
- Studies on effect of Structural modifications on Antimicrobial Chitoran
- In silico design,Synthesis and Invitrostudies of some novel 4H-Chromene Derivatives as Anticancer Agents
- Structural model of the Alpha-pPhosphoglucomutase and De Novo design of Inhibitors for the treatment of Mycobacterium Tuberculosis
Aza-lignan project in Medicinal Chemistry
Derivatives of Anticancer Agents
M. PHARMACY PROJECTS TOPICS – Pharmaceutical Chemistry
Synthesis of Biologically Active Lignan Natural Products via an Acyl-Claisen Rearrangement and an Unusual 1,4-diaryl Rearrangement
Studies towards the asymmetric synthesis of 1,4-benzodioxane neolignans
Development of bioactive 3C protease inhibitors as therapeutics to treat the common cold
Novel Selective Ligands of the CB1/D2 Receptor Heterodimer
Method Development for Characterization of Novel Copper Chelators in Patients with Diabetes
Synthetic investigation of neurologically active therapeutic agents
Synthesis of cyclic peptides isolated from a psychrophile
Total Synthesis of Aspergillus Spiroketal and its analogues
Asymmetric gold-catalysed synthesis of the paecilospirone spiroacetal
Total synthesis of lasionectrin and related analogues as novel anti-malaria agents
Synthesis of marine derived natural products Aigialospirol and its analogues
Total synthesis of terreinol, a spiroketal natural product, and investigation into enantioselective oxidative spiroketalisation
Studies towards onchidal’s acetycholine esterase inhibitory activity
The synthesis and investigation of marine natural products as potential anti-fouling agents
Bioactive marine natural product
The synthesis of natural product containing polymers for use in the prevention of biofilms
Marine Natural Products in Drug Discovery
Studies Towards the Design, Synthesis and Analysis of Bioactive Peptides
Studies towards the identification and synthesis of proteins expressed in intact and degenerate bovine cartilage
Peptide conjugation to build tumour selectivity into potential chemotherapeutic agents
Medicinal Chemistry Projects or Pharma Chemistry Projects for Masters / B Pharmacy
In Medicinal chemistry Projects B pharmacy and M Pharmacy students can take up wide variety of research topics which deals with Synthesis, Characterization and Docking Studies of some products, or Green Synthesis and Characterization of products, or In Silco Molecular Modeling or Cellular Redox State Modifications or High Throughput Kinetic Assay for Screening Potential Inhibitors. You can also try Method Development for Characterization of Novel products. Just you can make a list of your interested research topics for your B pharm and M Pharm projects and give them to your Guide. He or she will mentor you according to the current trend, necessity and resources availability.
Below are few examples of projects for pharmacy students who are interested in Medicinal Chemistry. These are the current trending and ongoing project list from different places and institutes.
- Synthesis of Mukanadin B and Analogues as Possible Neuroprotective Agents
- Synthesizing novel self assembled monolayer conductive polymers for improving biocompatible and norotrophic devices
- Synthesis of Biologically Active Lignan Natural Products via an Acyl-Claisen Rearrangement and an Unusual 1,4-diaryl Rearrangement
- Studies towards the asymmetric synthesis of 1,4-benzodioxane neolignans
- Total Synthesis of the Dineolignan Ophiocerol and Derivatives
- Synthesis of bioactive natural products and associated analogues
- Vaccine design for lectin targets
- Design and synthesis of rat selective toxicants
- Development of bioactive 3C protease inhibitors as therapeutics to treat the common cold
- Novel Selective Ligands of the CB1/D2 Receptor Heterodimer
- Method Development for Characterization of Novel Copper Chelators in Patients with Diabetes
- Synthetic investigation of neurologically active therapeutic agents
- Synthesis of cyclic peptides isolated from a psychrophile\
- Total Synthesis of Aspergillus Spiroketal and its analogues
- Asymmetric gold-catalysed synthesis of the paecilospirone spiroacetal \
- Total synthesis of lasionectrin and related analogues as novel anti-malaria agents
- Synthesis of marine derived natural products Aigialospirol and its analogues
- Total synthesis of terreinol, a spiroketal natural product, and investigation into
- enantioselective oxidative spiroketalisation
- Studies towards onchidal’s acetycholine esterase inhibitory activit
- Design and synthesis of rat selective toxicants
- Development of bioactive 3C protease inhibitors as therapeutics to treat the common cold
- Novel Selective Ligands of the CB1/D2 Receptor Heterodimer
- Method Development for Characterization of Novel Copper Chelators in Patients with Diabetes
- Synthetic investigation of neurologically active therapeutic agents
- Synthesis of cyclic peptides isolated from a psychrophile
- Total Synthesis of Aspergillus Spiroketal and its analogues
- Asymmetric gold-catalysed synthesis of the paecilospirone spiroacetal
- Total synthesis of lasionectrin and related analogues as novel anti-malaria agents
- Synthesis of marine derived natural products Aigialospirol and its analogues
- Total synthesis of terreinol, a spiroketal natural product, and investigation into enantioselective oxidative spiroketalisation
- Studies towards onchidal’s acetycholine esterase inhibitory activity.
Homology Modelling of Protein Steps Tools Software Tutorial PDF PPT Papers
What is Homology Modelling?
Homology modelling allows users to safely use rapidly generated in silico protein models in all the contexts where today only experimental structures provide a solid basis: structure-based drug design, analysis of protein function, interactions, antigenic behavior, and rational design of proteins with increased stability or novel functions. In addition, protein modeling is the only way to obtain structural information if experimental techniques fail. Many proteins are simply too large for NMR analysis and cannot be crystallized for X-ray diffraction.
Among the major approaches to three-dimensional (3D) structure prediction, homology modeling is the easiest one.
In the Homology Modelling, structure of a protein is uniquely determined by its amino acid sequence (Epstain, Goldberger, and Anfinsen, 1963). Knowing the sequence should, at least in theory, suffice to obtain the structure.
2. During evolution, the structure is more stable and changes much slower than the associated sequence, so that similar sequences adopt practically identical structures, and distantly related sequences still fold into similar structures. This relationship was first identified by Chothia and Lesk (1986) and later quantified by Sander and Schneider (1991). Thanks to the exponential growth of the Protein Data Bank (PDB), Rost (1999) could recently derive a precise limit for this rule. As long as the length of two sequences and the percentage of identical residues fall in the region marked as “safe,” the two sequences are practically guaranteed to adopt a similar structure.
Homology Modelling or Protein Modelling Example
Imagine that we want to know the structure of sequence A (150 amino acids long,). We compare sequence A to all the sequences of known structures stored in the PDB (using, for example, BLAST), and luckily find a sequence B (300 amino acids long) containing a region of 150 amino acids that match sequence A with 50% identical residues. As this match (alignment) clearly falls in the safe zone (Fig. 25.1), we can simply take the known structure of sequence B
(the template), cut out the fragment corresponding to the aligned region, mutate those amino acids that differ between sequences A and B, and finally arrive at our model for structure A. Structure A is called the target and is of course not known at the time of modeling.
Homology Modelling of Protein Steps Tools Software Tutorial PDF PPT
Homology Modelling Steps
In practice, homology modeling is a multistep process that can be summarized in seven steps:
1. Template recognition and initial alignment
2. Alignment correction
3. Backbone generation
4. Loop modeling
5. Side-chain modeling
6. Model optimization
7. Model validation
At almost all the steps choices have to be made. The modeler can never be sure to make the best ones, and thus a large part of the modeling process consists of serious thought about how to gamble between multiple seemingly similar choices. A lot of research has been spent on teaching the computer how to make these decisions, so that homology models can be built fully automatically. Currently, this allows modelers to construct models for about 25% of the amino acids in a genome, thereby supplementing the efforts of structural genomics projects.
Homology_Modelling – Protein PPT
Protein Homology modelling steps ppt Structures
Homology Modelling Steps, Homology Modelling Software, Homology Modelling Ppt, Homology Modelling Pdf, Homology Modeling Server, Protein Modelling Bioinformatics, Homology Modeling Tutorial, Homology Modelling Slideshare
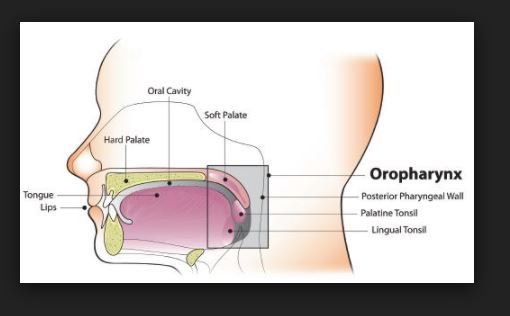
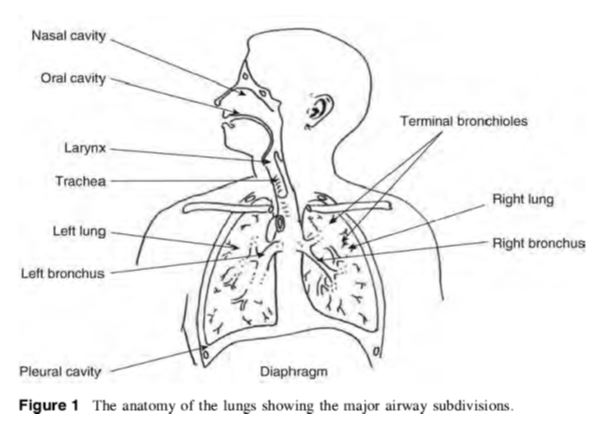
ANATOMY & PHYSIOLOGY Of Human Respiratory Tract
Anatomy and Physiology Of human respiratory system is a complicated organ system of very close structure– function relationships. The system consisted of two regions: the conducting airway and the respiratory region. The airway is further divided into many folds: nasal cavity and the associated sinuses, and the nasopharynx, oropharynx, larynx, trachea, bronchi, and bronchioles. The respiratory region consists of respiratory bronchioles, alveolar ducts, and alveolar sacs.
ANATOMY AND PHYSIOLOGY OF HUMAN RESPIRATORY TRACT:
The respiratory system works with the circulatory system to deliver oxygen from the lungs to the cells and remove carbon dioxide, and return it to the lungs to be exhaled. The exchange of oxygen and carbon dioxide between the air, blood and body tissues is known as respiration. Healthy lungs take in about 1 pint of air about 12–15 times each minute. All of the blood in the body is passed through the lungs every minute. The respiratory tract is divided into two main parts: the upper respiratory tract, consisting of the nose, nasal cavity and the pharynx; and the lower respiratory tract consisting of the larynx, trachea, bronchi and the lungs The trachea, which begins at the edge of the larynx, divides into two bronchi and continues into the lungs. The trachea allows air to pass from the larynx to the bronchi and then to the lungs. The bronchi divide into smaller bronchioles which branch in the lungs forming passageways for air. The terminal parts of the bronchi are the alveoli. The alveoli are the functional units of the lungs and they form the site of gaseous exchange
The blood barrier between the alveolar space and the pulmonary capillaries is very thin to allow for rapid gas exchange. During inspiration, oxygen diffuses through the alveoli walls and the interstitial space, into the blood. Carbon dioxide diffuses in the opposite direction during exhalation. Alveoli are small and there are approximately 300 million of them in each lung. Although alveoli are tiny structures, they have a very large surface area in total (~100 m2) for performing efficient gas exchange.
The alveoli form a honeycomb of cells around the spiral, cylindrical surface of the alveolar duct. The exposed alveolar surface is normally covered with a surface film of lipoprotein material.
There are several types of pulmonary alveolar cells. Type I (or small type A), are non-phagocytic, membranous pneumocytes. These surface-lining epithelial cells are approximately 5 μm in thickness and possess thin squamous cytoplasmic extensions that originate from a central nucleated portion. These portions do not have any organelles and hence they are metabolically dependent on the central portion of the cell. This reduces their ability to repair themselves if damaged. Attached to the basement membrane are the larger alveolar cells (Type II, type B or septal cells). These rounded, granular, epithelial pneumocytes are approximately 10 to 15 μm tick. There are 6 to 7 cells per alveolus and these cells possess great metabolic activity. They are believed to produce the surfactant material that lines the lung and to be essential for alveolar repair after damage from viruses or chemical agents.
Amongst, the important roles of the lungs, one can cite: (i) supply oxygen, (ii) remove wastes and toxins, and (iii) defend against hostile intruders. The lungs have three dozen distinct types of cells. Some of these cells scavenge foreign matter. Others have cilia that sweep the mucous membranes lining the smallest air passages. Some cells act on blood pressure control, while others spot infection invaders.
The respiratory system is susceptible to a number of diseases, and the lungs are prone to a wide range of disorders caused by genetic factors, infection and pollutants in the air. The most common problems of the respiratory system are:
- Asthma
- Bronchiolitis
- Chronic obstructive pulmonary disease (COPD)
- Common cold
- Cough
- Cystic fibrosis (CF)
- Lung cancer
- Pneumonia
- Pulmonary hypertension
PRINCIPAL MECHANISMS OF RESPIRATORY DEPOSITION
The deposition of inhaled particles in the different regions of the respiratory system is very complex, and depends on many factors. Some of the factors influencing respiratory deposition include:
- Breathing rate
- Mouth or nose breathing
- Lung volume
- Respiration volume
- Health of the individual
- Bifurcations in the airways result in a constantly changing hydrodynamic flow field.
Depending on the particle size, airflow, and location in the respiratory system, particle deposition occurs via on of the following principal mechanisms:
Impaction
Each time the airflow changes due to a bifurcation in the airways, the suspended particles tend to travel along their original path due to inertia and may impact on an airway surface. This mechanism is highly dependent on aerodynamic diameter, since the stopping distance for very small particles is quite low. Impaction occurs mostly in the case of larger particles that are very close to airway walls, near the first airway bifurcations. Therefore, deposition by impaction is greatest in the bronchial region. Impaction accounts for the majority of particle deposition on a mass basis.
Sedimentation
Sedimentation is the settling out of particles in the smaller airways of the bronchioles and alveoli, where the air flow is low and airway dimensions are small. The rate of sedimentation is dependent on the terminal settling velocity of the particles, so sedimentation plays a greater role in the deposition of particles with larger aerodynamic diameters. Hygroscopic particles may grow in size as they pass through the warm, humid air passages, thus increasing the probability of deposition by sedimentation.
Interception
Interception occurs when a particle contacts an airway surface due to its physical size or shape. Unlike impaction, particles that are deposited by interception do not deviate from their air streamlines. Interception is most likely to occur in small airways or when the air streamline is close to an airway wall. Interception is most significant for fibers, which easily contact airway surfaces do to their length. Furthermore, fibers have small aerodynamic diameters relative to their size, so they can often reach the smallest airways.
Diffusion
Diffusion is the primary mechanism of deposition for particles less than 0.5 microns in diameter and is governed by geometric rather than aerodynamic size. Diffusion is the net transport of particles from a region of high concentration to a region of lower concentration due to Brownian motion. Brownian motion is the random wiggling motion of a particle due to the constant bombardment of air molecules. Diffusional deposition occurs mostly when the particles have just entered the nasopharynx, and is also most likely to occur in the smaller airways of the pulmonary (alveolar) region, where air flow is low.
Absorption – bioavailability of drugs
Although inhaled drugs have been used for over 50 years to treat airway disease and are in development or being considered for the treatment of many other lung diseases, insulin is at present time the only one representative inhaled drug on the market for systemic disease. Exubera® (insulin human [rDNA origin] inhalation powder is the first diabetes treatment which can be inhaled. Exubera® helps control high blood sugar, works in adults with type 1 diabetes and with type 2 diabetes as well This therapeutic success has lead a number of other companies to investigate and to advance clinical trials as inhaled formulations for systemic applications with a variety of large molecules (leuprolide, a luteinizing hormone-releasing hormone (LHRH) analogue, …). Recent advances in the development of particle technologies and devices now make it possible to formulate, stabilize, and accurately deliver almost any drug to the lungs.
The pulmonary membrane is naturally permeable to small molecule drugs and to many therapeutic peptides and proteins. The epithelium of the lung, the significant barrier to absorption of inhaled drugs, is thick (50–60 μm) in the trachea, but diminishes in thickness to an extremely thin 0.2 μm in the alveoli. The change in cell types and morphology going from trachea, bronchi, and bronchioles to alveoli is very dramatic. The lungs are for more permeable to macromolecules than any other portal of entry into the body. Some of the most promising therapeutic agents are peptides and proteins, which could be inhaled instead of injected, thereby improving compliance .Particularly, peptides that have been chemically altered to inhibit peptidase enzymes exhibit very high bioavailabilities by the pulmonary route .Indeed, natural mammalian peptides, les than 30 amino acids (somatostatin, vaso active intestinal peptide [VIP], and glucagons), are broken down in the lung by ubiquitous peptidases and have very poor bioavailabilities. Conversely, proteins with molecular weights between 6000 and 50,000 Da are relatively resistant to most peptidases and have good bioavailabilities following inhalation. For larger proteins, the bioavailabilities and absorption mechanisms are not well completely elucidated.
ADVANTAGES OF PULMONARY DRUG DELIVERY SYSTEM
- The ability to nebulize viscous drug formulations for pulmonary delivery, thereby overcoming drug solubility issues with the ability to use lipid, water or lipid/water emulsions as drug carriers.
- Ability to nebulize viscous liquids into droplets in the 2-5μm range regardless of the carrier composition solubility which would allow for a wide range of drug formulation options.
- Increased drug delivery efficacy due to size-stable aerosol droplets with reduced
hygroscopic growth and evaporative shrinkage.
- Liposomal drug formulations remain stable when nebulized.
- Ability to nebulize protein-containing solutions.
- For hand held inhaler applications, drug does not need to be emulsified in liquefied nebulizing gas to achieve aerosolization.
Pharmaceutics M Pharmacy Project Title – Example Summary Aim – B pharm Projects
BIOAVAILABILITY STUDY AND COLONIC RESIDENCE TIME EVALUATION BY X-RAY OF ORNIDAZOLE FROM COATED TABLETS IN HEALTHY HUMAN VOLUNTEERS
Pharmaceutics M Pharmacy Project Title – Example Summary Aim – B pharm Projects
BIOAVAILABILITY STUDY AND COLONIC RESIDENCE TIME EVALUATION BY X-RAY OF ORNIDAZOLE FROM COATED TABLETS USING APPROVED PHARMACEUTICAL EXCIPIENTS IN HEALTHY HUMAN VOLUNTEERS
Summary
Aim : 1) To carry bioavailability study of Ornidazole from coated tablets by using pharmaceutical excipients and compare with marketed product.
2) To carry colonic residence time evaluation by X-ray study of Ornidazole from coated tablets.
Drugs used : Ornidazole 400 mg.
Subjects : Eight healthy human male volunteers
Study design : Crossover design
Institution :
Principal Investigator:
Study Procedure:
Eight human healthy male subjects in the age group of 25-30 will be enrolled in the study after physical examination by a physician and standard laboratory tests.
Inclusion Criteria:
- Non-allergic to drug
- Healthy as per the physical examination and laboratory tests
- Non-participation in any study/blood donation during preceding three months
- Written informed consent
Study design: Simple randomized crossover design
The subject will be treated with single oral dose of Ornidazole after overnight fasting. In the crossover study, subjects will be given coated tablets of Ornidazole. Blood samples will be collected at 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24 and 30 hours.
The subject will be treated with single oral dose of placebo tablets after overnight fasting. In the crossover study, subjects will be given placebo tablets of Ornidazole. X-Rays will be taken at 2, 5, 8,12 and 24 hours.
Pharmaceutics M Pharmacy Project Title – Example Summary Aim – B pharm Projects PDF
Pharmaceutics M Pharmacy Project Title – Example Summary Aim – B pharm Projects 
Treatments: Eight male volunteers shall be distributed in to two groups. A 2×2 cross over design shall be used in the study. Each volunteer in the two groups will receive the floating matrix tablets and commercial dosage form as .
The study consists of two treatments (Ornidazole coated, commercial). Ornidazole 400 mg will be given by oral route in the form of coated tablets and blood samples will be collected at 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24 and 30 hours.
A drug free interval of at least two weeks will be kept between the two treatments. A standard breakfast will be served 2 hours after drug administration followed by standard lunch after 4 hours.
ORNIDAZOLE
Ornidazole is an anti infective / antibacterial and antiprotozaol drug available as 400mg, 500 mg and 1000 mg tablets for oral administration. Its chemical name is 1-(3-chloro-2-hydroxypropyl)-2-methyl-5-nitroimidazole.
The half-life of the drug is approximately 7.4 hours in plasma. Ornidazole is metabolised in liver through biotranformation reactions while excretion is mainly by Urine.
ContraIndications:
Hypersensitivity to ornidazole or to other nitroimidazole derivatives
Adverse Reactions:
Somnolence, headache, nausea, vomiting, dizziness, tremor, rigidity, poor coordination, seizures, tiredness, vertigo, temporary loss of consciousness and signs of sensory or mixed peripheral neuropathy, taste disturbances, abnormal LFTs, skin reaction.
Physical properties:
Solubility : It is slightly soluble in water, and soluble in chloroform.
Pka : 2.4 ± 0.1
Category : It is a anti-infective and anti-protozoal agent
Pharmacokinetics
Bioavailability : >90 % by oral route
Absorption : Absorbed from entire GIT.
Protein Binding : <15 %
Half life : 14.67 + 1.0 hrs
Dosage : 400 to 1000 mg daily.
APPROVAL OF THE ETHICAL COMMITTEE
The study entitled “Bioavailability study and colonic residence time evaluation by x-ray of Ornidazole from coated tablets in healthy human volunteers” has been approved / not approved for conducting in the healthy human volunteers.
[PDF PPT DOC] Pharmaceutical FILTER VALIDATION – Sterile Protocol FDA Guide
FILTER VALIDATION
Do you know Pharmaceutical Filter validation importance? Pharmaceutical processes are validated processes to assure a reproducible product within set specifications. Equally important is the validation of the filters used within the process, especially the sterilizing grade filters, which, often enough, are used before filling or the final processing of the drug product. In its Guideline on General Principles of Process Validation, 1985, and Guideline on Sterile Drug Products Produced by Aseptic Processing, 1987, the FDA makes plain that the validation of sterile processes is required by the manufacturers of sterile products. Sterilizing grade filters are determined by the bacteria challenge test. This test is performed under strict parameters and a defined solution (ASTM F 838-83).
In any case, the FDA nowadays also requires evidence that the sterilizing grade filter will create a sterile filtration, no matter the process, fluid or bioburden, found. This means that bacteria challenge tests have to be performed with the actual drug product, bioburden, if different or known to be smaller than B. diminuta and the process parameters. The reason for the requirement of a product bacteria challenge test is threefold. First, the influence of the product and process parameters to the microorganism has to be tested. There may be cases of either shrinkage of organisms due to a higher osmolarity of the product or prolonged processing times. Second, the filter’s compatibility with the product and the parameters has to be tested. The filter should not show any sign of degradation due to the product filtered. Additionally, rest assurance is required that the filter used will withstand the process parameters; e.g., pressure pulses, if happening, should not influence the filter’s performance.
Third, there are two separation mechanisms involved in liquid filtration: sieve retention and retention by adsorptive sequestration. In sieve retention, the smallest particle or organism size is retained by the biggest pore within the membrane structure. The contaminant will be retained, no matter the process parameters. This is the ideal. Retention
by adsorptive sequestration depends on the filtration conditions. Contaminants smaller than the actual pore size penetrate such and may be captured by adsorptive attachment to the pore wall. This effect is enhanced using highly adsorptive filter materials, for example,
Glassfibre as a prefilter or Polyamide as a membrane. Nevertheless, certain liquid properties can minimize the adsorptive effect, which could mean penetration of organisms. Whether the fluid has such properties and will lower the effect of adsorptive sequestration and may eventually cause penetration has to be evaluated in specific product bacteria challenge tests.
Before performing a product bacteria challenge test, it has to be assured that the liquid product does not have any detrimental, bactericidal or bacteriostatic, effects on the challenge organisms. This is done utilizing viability tests. The organism is inoculated into the product
to be filtered at a certain bioburden level. At specified times, the log value of this bioburden is tested. If the bioburden is reduced due to the fluid properties, a different bacteria challenge test mode becomes applicable. If the reduction is a slow process, the challenge test will
be performed with a higher bioburden, bearing in mind that the challenge level has to reach 107 per square centimeter at the end of the processing time. If the mortality rate is too high, the toxic substance is either removed or product properties are changed. This challenge fluid is called a placebo. Another methodology would circulate the fluid product through the filter at the specific process parameters as long as the actual processing time would be. Afterwards, the filter is flushed extensively with water and the challenge test, as described in ASTM F838-38, performed. Nevertheless, such a challenge test procedure would be more or less a filter compatibility test.
Besides the product bacteria challenge test, tests of extractable substances or articulate releases have to be performed. Extractable measurements and the resulting data are available from filter manufacturers for the individual filters. Nevertheless, depending
on the process conditions and the solvents used, explicit extractable tests have to be performed. These tests are commonly done only with the solvent used with the drug product but not with the drug ingredients themselves, because the drug product usually
covers any extractables during measurement. Such tests are conducted by the validation services of the filter manufacturers using sophisticated separation and detection methodologies, as GC-MS, FTIR, and RP-HPLC. These methodologies are required, due to the fact that the individual components possibly released from the filter have to be identified and quantified. Elaborate studies, performed by filter manufacturers, showed that there is neither a release of high quantities of extractables (the range is ppb to max ppm per 10-inch element) nor have toxic substances been found. Particulates are critical in sterile filtration, specifically of injectables. The USP 24 (United States Pharmacopoeia) and BP (British Pharmacopoeia) quote specific limits of particulate level contaminations for defined particle sizes. These limits have to be kept and, therefore, the particulate release of sterilizing
grade filters has to meet these requirements. Filters are routinely tested by evaluating the filtrate with laser particle counters. Such tests are also performed with the actual product under process conditions to prove that the product, but especially process conditions, do
not result in an increased level of particulates within the filtrate.
Additionally, with certain products, loss of yield or product ingredients due to adsorption shall be determined. For example, preservatives, like benzalkoniumchloride or chlorhexadine, can be adsorbed by specific filter membranes. Such membranes need to be saturated by the preservative to avoid preservative loss within the actual product. This preservative loss e.g., in contact lens solutions, can be detrimental, due
to long-term use of such solutions. Similarly, problematic would be the adsorption of required proteins within a biological solution. To optimize the yield of such proteins within an application, adsorption trials have to be performed to find the optimal membrane
material and filter construction.
Cases that use the actual product as a wetting agent to perform integrity tests require the evaluation of product integrity test limits. The product can have an influence on the measured integrity test values due to surface tension, or solubility. A lower surface tension,
for example, would shift the bubble point value to a lower pressure and could result in a false negative test. The solubility of gas into the product could be reduced, which could result in false positive diffusive flow tests. Therefore, a correlation of the product as a wetting agent to the, water wet values has to be done, according to standards set by the manufacturer of the filter. This correlation is carried out by using a minimum of three filters of three filter lots. Depending on the product and its variability, one or three product lots are used to perform the correlation. The accuracy of such a correlation is enhanced by automatic integrity test
machines. These test machines measure with highest accuracy and sensitivity and do not rely on human judgement, as with a manual test. Multipoint diffusion testing offers the ability to test the filter’s performance and, especially, to plot the entire diffusive flow graph through the bubble point. The individual graphs for a water-wet integrity test can now be compared to the product wet test and a possible shift evaluated. Furthermore, the multipoint diffusion test enables the establishment of an improved statistical base to determine the product wet versus water-wet limits.
Look out here Pharmaceutical FILTER INTEGRITY TESTING – FDA Guideline on Sterile Drug Products
Pharmaceutical Filter Validation References:
- Cooper and Gunn’s. Tutorial Pharmacy by S.J.Carter.
- Pharmaceutical engineering; K. Sambamurthy
- Pharmaceutical engineering; principles and practices, C.V.S. Subrahmanyam
- Encyclopedia of pharmaceutical technology, vol 3, edited by James Swarbrick.
- Pikal, M.J.; Lukes, A.L.; Lang, J.E. Thermal decomposition of amorphous beta-lactam antibacterials. J. Pharm. Sci. 1977, 66, 1312–1316.
- Pikal, M.J.; Lukes, A.L.; Lang, J.E.; Gaines, K. Quantitative crystallinity determinations of beta-lactam antibiotics by solution calorimetry: correlations with stability. J. Pharm. Sci. 1978, 67, 767–773.
- Pikal, M.J.; Dellerman, K.M. Stability testing of pharmaceuticals by high-sensitivity isothermal calorimetry at 25_C: cephalosporins in the solid and aqueous solution states. Int. J. Pharm. 1989, 50, 233–252.
- Incoming searches:
- filter validation ppt,
filter validation pdf,
brevundimonas diminuta filter validation,
filter validation millipore,
filter validation fda,
sterile filtration validation protocol,
bacterial retention testing of sterilizing-grade filters,
sterile filtration definition,
Pharmaceutical Filter validation pdf:
Pharmaceutical filter validation ppt filter validation power point
Pharmaceutical filter validation ppt filter
Pharmaceutical filter validation ppt filter validation pdf
Pharmaceutical filter validation ppt filter validation power point
{PDF PPT DOC} FILTRATION EQUIPMENT – Filtration Mechanism & Types – Adv + Disadvantages
{PDF PPT DOC} FILTRATION EQUIPMENT – Filtration Mechanism & Types – Adv + Disadvantages deals with details of filtration, filtration equipment,definitions, mechanism of filtration, Classification of filtration equipment, Different types of filtration its advantages & Disadvantages.
FILTRATION Definition:
FILTRATION may be defined as the separation of a solid from a fluid by means of a porous medium that retains the solid but allows the fluid to pass.
The term fluid includes liquids and gases, so that both these may be subjected to filtration.
The suspension of solid and liquid to be filtered is known as the “slurry”. The porous medium used to retain the solids is described as the filter medium; the accumulation of solids on the filter is referred to as the filter cake, while the clear liquid passing through the filter is the filtrate.
2.MECHANISMS OF FILTRATION:
The mechanisms whereby particles are retained by the filter are of significance only in the early stages of liquid filtration, as a rule. Once a preliminary layer of particles has been deposited, the filtration is effected by the filter cake, the filter medium serving only as a support.
STRAINING:
The simplest filtration procedure is “straining”, in which, like sieving, the pores are smaller than the particles, so that the latter are retained on the filter medium.
ENTANGLEMENT:
If the filter medium consists of a cloth with a nap or a porous felt, then particles become entangled in the mass of fibres. Usually the particles are smaller than the pores, so that it is possible that impingement is involved.
ATTRACTIVE FORCES:
In certain circumstances, particles may collect on a filter medium as a result of attractive forces. The ultimate in this method is the electrostatic precipitator, where large potential differences are used to remove the particles from air streams.
In practise, the process may combine the various mechanisms, but the solids removal is effected normally by a straining mechanism once the first complete layers of solids has begun to form the cake on the filter medium.
3.CLASSIFICATION OF THE FILTRATION EQUIPMENT:
Equipment’s are classified based on the application of external force.
- Pressure filters: plate and frame filter press and metafilter
- Vacuum filters: filter leaf
- Centrifugal filters
Classification based on the operation of the filtration
- Continuous filtration: discharge and filtrate are separated steadily and uninterrupted
- Discontinuous filtration: discharge of filtered solids is intermittent. Filtrate is removed continuously. The operation must be stopped to collect the solids.
Classification based on the nature of filtration
- Cake filters: remove large amounts of solids (sludge or crystals)
- Clarifying filters: remove small amounts of solids
- Cross-flow filters: feed of suspension flows under pressure at a fairly high velocity across the filter medium.
Equipment’s of pharmaceutical interest:
- Sand filters:
- Filter presses: chamber, plate and frame filters ( non-washing/washing; closed delivery/open delivery)
- Leaf filters
- Edge filters: stream line and meta filters
- Rotary continuous filters
- Membrane filters
4.1.SAND FILTERS
These are used mainly when relatively small amounts of solid are to be removed from the liquid and when relatively large volumes of liquid must be handled at minimum cost. A standardised pressure sand filter consists of a cylindrical tank at the bottom of which are a number of brass strainers which are either mounted on a false bottom or connected to a manifold embedded in concrete. The strainers have narrow slots sawed in them. Over the strainers is a layer of several inches of moderately coarse gravel on the top of which is a 2 to 4 ft. deep sand layer that forms the actual filter medium. The water to be filtered is introduced at the top on to a baffle which prevents disturbance in the sand by a direct stream. The filtered water is drawn off through the strainers at the bottom. When the precipitate clogs the sand to the extent of retarding the flow of water, it is removed by back washing. This operation consists of introducing water through the strainers, so that it may flow up through the sand bed and-out through the connection that is normally the inlet. This wash water is wasted. These sand filters are applicable only to the separation of precipitates that can be removed from the sand in this manner and that are to be discarded. Gelatinous precipitates or precipitates that coat the sand so that they cannot be removed by back washing or precipitates that are to be recovered cannot be handled in the sand filter.
Capacity is usually 2 to 4 gpm/sq.ft of surface of filtering area.
Fig1: pressure sand filter
For filtering excessively large quantities of very clean water, an open or rapid sand filter is used. It is similar to the pressure sand filter except that the sand is contained in large, open concrete boxes instead of in a closed pressure tank. Sand filter used in this way becomes a gravity filter (also called hydrostatic head filter).
ADVANTAGES:
Gravity filters have advantages of extreme simplicity, needing only simple accessories, low first cost and can be made of almost any material.
DISADVANTAGES:
- Relatively low rate of filtration.
- Excessive floor area needed and high labour charges
- If the amount of particulate matter to be removed is too small or it is finely divided, sand filter will not remove the suspended solids.
- In processes involving organic materials there may be danger of bacterial infection from an infected process-water supply and the sand filter cannot remove the bacteria as such. In these cases a coagulant like ferrous sulphate or aluminium sulphate is added to the water before filtration. These are hydrolysed by the alkalinity of most normal waters with the formation of a flocculant precipitate of iron or aluminium hydroxide. This precipitate adsorbs finely divided suspended matter and even bacteria, even if added to the water in very small amounts. The resultant flocs, though fine, are removed by the sand filters.
4.2.PLATE AND FRAME FILTER PRESS:
.
Principle : The mechanism is surface filtration. The slurry enters the frame by pressure and flows through the filter medium: The filtrate is collected on the plates and sent to the outlet. A number of frames and plates are used so that surface area increases and consequently large volumes of slurry can be processed simultaneously with or without washing.
Construction .: The construction of a plate and frame filter press is shown in the figure2. The filter press is made of two types of units, plates and frames.
(a) Frame-Maintains the slurry reservoir, inlet (eye) for slurry.
(b) Filter medium.
(c) Plate along with section-supports the filter medium, receiving the filtrate and outlet (eye).. (d) Assemb1y of plate and frame filter press.
These are usually made of aluminium alloy. Sometimes these are also lacquered for protection against corrosive chemicals and made suitable for steam sterilisation.
Frame contains an open space inside wherein the slurry reservoir is maintained for filtration and an inlet to receive the slurry. It is indicated by two dots in the description (Figure ).The plate has a studded or grooved surface to support the filter cloth and an outlet. It is indicated by one dot in the description (Figure ). The filter medium (usually cloth) is interposed between plate and frame.
Frames of different thicknesses are available. It is selected based on the thickness of the cake formed during filtration. Optimum thickness of the frame should be chosen. Plate, filter medium, frame, filter medium and plate are arranged in the sequence and clamped to a supporting structure. It is normally described by dots as 1.2.1.2.1 so on. A number of plates and frames are employed so that filtration area is a large as necessary. In other words, a number of filtration units are operated in parallel. Channels for the slurry inlet and filtrate outlet can be arranged by fitting eyes to the plates and frames, these join together to form a channel. In some types, only one inlet channel is formed, while each plate is having individual outlets controlled by valves.
Working : The working of the frame and plate process can be described in two steps, namely filtration and washing of the cake (if desirable).
Filtration operation : The working of a plate and frame press is shown in Figure. Slurry enters the frame (marked by 2 dots) from the feed channel and passes through the filter medium on to the surface of the plate (marked by I dot). The solids form a filter cake and remain in the frame. The thickness of the cake is half of the frame thickness, because on each side of the frame filtration occur. Thus, two filter cakes are formed, which meet eventually in the centre of the frame. In general, there will be an optimum thickness of filter cake for any slurry, depending on the solid content in the slurry and the resistance -of the
filter cake.
The filtrate drains between the projections on the surface of the plate and escapes from the outlet. As filtration proceeds, the resistance of the cake increase and the filtration rate decreases. At a certain point, is preferable to stop the process rather than continuing at very low flow rates. The press is emptied and the cycle is restarted.
Fig 3: plate and frame filter press
Washing operation: If it is necessary to wash the filter cake, the ordinary plate and frame press is unsatisfactory. Two cakes are built up in the frame meeting eventually in the middle. This means that flow is brought virtually to a stand still. Hence, water wash using the same channels of the filtrate is very inefficient, if not impossible. A modification of the plate and frame press is used. For this purpose, an additional channel is included (Figure). These wash plates are identified by three dots. In half the wash plate there is a connection from the wash water channel to the surface of the plate.
The sequence of arrangement of plates and frames can be represented by dQts as 1.2.3.2.1.2.3.2.1.2.3.2.1 so on (between I and 1,2.3.2 must be arranged). Such an arrangement is shown in Figure (a) and (b) for the operations of filtration and water washing, respectively.
The steps are as follows.
(1) Filtration proceeds in the ordinary way until the frames are filled with cake.
(2) To wash the filter cake, the outlets of the washing plates (three dots) are closed.
(3) Wash water is pumped into the washing channel. The water enters through the inlets on to the surface of the washing (three dots) plates.
(4) Water passes through the filter cloth and enters frame (two dots) which contains the cake. Then water washes the cake, passes through the filter cloth and enters the plate (one dot) down the surface.
(5)Finally washed water escapes through the outlet of that plate.
Fig 4: plate and frame filter press with water wash facility
Thus with the help of special washing plates, it is possible for the wash-water to flow over the entire surface of washing (three dots) plate, so that the flow resistance of the cake is equal to all points. Hence, the entire cake is washed with equal efficiency.
Fig 5: principles of filtration and washing
It should be noted that water- wash is efficient only if the frames are full with filter cake. If the solids do not fill the frame completely, the wash water causes the cake to break (on the washing plate side of the frame) then washing will be less effective. Hence, it is essential to allow the frames become completely filled with the cake. This helps not only in emptying the frames but also helps in washing the cake correctly.
Special provisions:
(I) Any possible contamination can be observed by passing the filtrate through a glass tube or sight glass from the outlet on each plate. This permits the inspection of quality of the filtrate. The filtrate goes through the control valve to an outlet channel.
(2) The filtration process from each plate can be seen. In the event of a broken cloth, the faulty plate can be isolated and filtration can be continued with one plate less.
Uses : Filter sheets composed of asbestos and cellulose are capable of retaining bacteria, so that sterile filtrate can be obtained, provided that the whole filter press and filter medium have been previously sterilized. Usually steam is passed through the assembled unit for sterilization.
Examples include collection of precipitated antitoxin, removal of precipitated proteins from insulin liquors and removal of cell broth from the fermentation medium.
Heating/cooling coils are incorporated in the press so as to make it suitable for the filtration of viscous liquids .
Advantages :
(1) Construction of filter press is very simple and a variety of materials can be used.
– Cast iron for handling common substances.
— Bronze for smaller units.
– Stainless steel is used there by contamination can be avoided.
– Hard rubber or plastics where metal must be avoided.
– Wood for lightness though it must be kept wet.
(2) It provides a large filtering area in a relatively small floor space. It is versatile, the capacity being variable according to the thickness of frames and the number used. Surface area can be increased by employing chambers up to 60.
(3) The sturdy construction permits the use of considerable pressure difference. About 2000 kilopascals can’ be normally used.
(4) Efficient washing of the cake is possible.
(5) Operation and maintenance is straight forward, because there are no moving parts, filter cloths are easily renewable. Since all joints are external, a plate can be disconnected if any leaks are visible. Thus contamination of the filtrate can be avoided.
(6) It produces dry cake in the form of slab.
Disadvantages :
(I)it is a batch filters so there is a good deal of ‘down-time’, which is non-productive.
(2) The filter press is an expensive filter. The emptying time, the labour involved and the wear and tear of the cloth resulting in high costs.
(3)operation is critical, as the frames should be full, otherwise washing is inefficient and the cake is difficult to remove.
(4) The filter press is used for slurries containing less than 5% solids. So high costs make it imperative that this filter press is used for expensive materials. Examples include the collection of precipitated antitoxin and removal of precipitated proteins from insulin liquors.
4.3.FILTER LEAF:
The filter leaf is probably the simplest form, of filter, consisting of a frame enclosing a drainage screen or grooved plate, the whole unit being covered with filter cloth. The outlet for the filtrate connects to the inside of the frame. The frame may be of any shape, circular, square or rectangular shapes being used in practice. In use, the filter leaf is
immersed in the slurry’ and a receiver and vacuum system connected to the filtrate outlet. The method has the advantage that the slurry can be filtered from any vessel and the cake can be washed simply by immersing the filter, in a vessel of water. Removal of the cake is facilitated by the use of reverse air flow.
An alternative method is to enclose the filter leaf in a special vessel into which the slurry is pumped under pressure.
This form is commonest in filters where a number of leaves are connected to common outlet, to provide a larger area for filtration. A typical example is “ the Sweetland filters“
Fig 6: filter leaf Fig 7: sweetland filter
The filter leaf is a versatile piece of equipment. Area can be varied by employing a suitable number of units, and the pressure difference may be obtained with vacuum or by using pressures up to order of 8 bars. The leaf filter is most satisfactory if the solids content of slurry is not too high, about 5 per cent being a suitable maximum. A higher proportion, results in excessive non-productive time while the filter being emptied and, provided this is observed. Labour costs for operating the filter are comparatively moderate·
The special feature of the leaf filter is the high efficiency of washing; in fact the cake can be dislodged and refiltred from the wash water if desired.
4.4.ROTARY FILTER:
Filters such as the filter leaf and filter press are batch operated and can handle dilute suspensions only, if the process is to be economic. In large scale operation, continuous operation is sometimes desirable and it may be necessary to filter slurries containing a high proportion of solids.
The rotary filter is continuous in operation and has a system for removing the cake that is formed, hence it is suitable for use with concentrated slurries.
The rotary filter consists of a number of filter units (usually 16-20 ) arranged so that the units are passing in continuous succession through the various stages.
One form is the rotary disc filter in which the sectors shaped filter leafs form a disc with the outlet from the each leaf connected to the vacuum system, compressed air, and the appropriate receivers, in the correct sequence, by means of special rotating valve.
fig 8: Rotary drum filter
The commonest form in use in the pharmaceutical industry, however, is the rotary drum filters, a section of which is shown in figure, from which it will be seen that the filter units have the shape of longitudinal segments of the pheriphery of a cylinder. Thus, each filter unit is rectangular in shape with a curved profile so that a number can be joined up to form a drum. Each unit has a perforated metal surface to the outer part of the drum and is covered with filter cloth. Appropriate connections are again made from each unit through a rotating valve at the center of the drum. In operation, the drum rotates at low speed, so that cach unit passes through the various zones shown in figure and listed in table.
Rotary filters may be up to 2m in diameter and 3.5m in length, giving areas of the order of 20m2. Special attachments may be included for special purposes; for example if the cake shrinks and cracks as it dries out, cake compression rollers can be fitted. These compress the cake to a homogenous mass to improve the efficiency of washing as the cake passes through the washing zone, or to aid drainage of wash water as the cake passes to the drying zone.
Where the solids of the slurry are such that the filter cloth becomes blocked with the particles, a pre coat filter may be used. This is variant in which a precoat of filter aid is deposited on the drum prior to the filtration process. The scraper knife then removes the solid filtered from the slurry together with a small amount amount of the precoat, the knife advancing slowly as the precoat is removed.
If the removal of the cake presents the problems, alternative discharge methods can be used. The string discharge rotary filter, for example, is especially useful for certain pharmaceutical applications, particularly for filtering the fermentation liquor in the manufacture of antibiotics where the mould is difficult to filter by ordinary methods because it forms a felt-like cake. The string discharge filter is operated by means of a number of loops of string which pass the drum, and cause the cake to form over the strings as shown in the diagram. The strings are in contact with the surface of the drum up to the cake removal zone, where they leave the surface and pass over additional small rollers before returning to again contact the drum. In operation, the strings lift the filter cake of the filter medium, and the cake is broken by the sharp bend, over the rollers so that it is easily collected while the strings return to the drum.
Advantages:
(a) The rotary filter is automatic and is continuous in operation, so that labour costs are very low
(b) the filter has a large capacity, in fact, the area of the filter as represented by A of darcy’s law is infinity.
(c) variation of the speed of rotation enables the cake thickness to be controlled and for solids that form an impermeable cake, the thickness may be limited to less than 5mm. On the other hand, if the solids are coarse, forming a porous cake, the thickness may be 100mm or more.
Table 1: various zones in rotary filter.
Fig 9: string discharge rotary drum filter
Disadvantages:
- The rotary filter is a complex piece of equipment with many moving parts and is very expensive and in addition to the filter itself, ancillary equipments such as vacuum pumps and vacuum receivers and traps, slurry pumps and agitators are required.
- The cake tends to crack due to the air drawn through by the vacuum system so that washing and drying are not efficient.
- Being a vaccum filter the pressure difference is limited to 1 bar and hot filtrates may boil.
- The rotary filter is suitable only for straight forward slurries,being less satisfactory if the solids formed an impermeable cake or will not separate cleanly from the cloth.
USES OF THE ROTARY FILTERS:
The rotary filter is most suitable for continuous operation on large quantities of slurry, especially if the slurry contains considerable amounts of solids, i.e., in the range 15-30%.
Examples of pharmaceutical applications include the collection of calcium carbonate, magnesium carbonate and starch, and the separation of mycelium from tyhe fermentation liquor in the manufacture of antibiotics.
4.5.MEMBRANE FILTERS:
These are plastic membranes based on cellulose acetate, cellulose nitrate or mixed cellulose esters with pore sizes in the micron or submicron range. They are very thin (about 120 micron thick) and must be handled carefully. They act like a sieve trapping particulate matter on their surface.
Several grades of filters are available with pore sizes ranging from 0.010 ± 0.002
micron to 5.0 ± 1.2 micron. Type codes VF and SM are given by Millipore Filter Corp. for
these two extreme ranges respectively.
Filters with pore sizes from 0.010 to 0.10 micron can remove virus particles from water or air. Filters with pore sizes from 0.30 to 0.65 microns are employed for removing bacteria. Filters with the larger pore sizes, viz. 0.8, 1.2 and 3.0 to 5.0 microns are employed, for example, in aerosol, radio activity and particle sizing applications.
During use membrane filters are supported on a rigid base of perforated metal, plastic or coarse sintered glass as in the case of fibrous pad filters. If the solution to be filtered contains a considerable quantity of suspended matter, preliminary filtration through a suitable depth filter avoids clogging of the membrane filter during sterile filtration. They are brittle when dry and can be stored indefinitely in the dry state but are fairly tough when wet.
ADVANTAGES:
- No bacterial growth through the filter takes place during prolonged filtration.
- They are disposable and hence no cross contamination takes place.
- Adsorption is negligible they yield no fibres or alkali into the filterate. Filtration rate is rapid.
DISADVANTAGES:
- They may clog though rarely.
- Ordinary types are less resistant to solvents like chloroform
4.6.EDGE FILTERS:
A form of filters that differs markedly from those described above is the type known generally as edge filters. Filters such as the leaf or press act by presenting a surface of the filter medium to the slurry. Edge filters use a pack of the filter medium, so that filtration occurs on the edges. Forms using packs of media such as filter paper can be used but in the pharmaceutical industry greatest use is made of the Metafilter.
4.7.METAFILTER:
The metafilter, in its simplest form, consists of a grooved drainage rod on which is packed a series of metal rings. These rings, usually of stainless steel, are about 15mm inside diameter, and 0.8mm in thickness, with a number of semi-circular projections on one surface, as shown in the figure. The height of the projections and the shape of the section of the ring are such as that when the rings are packed together, all the same way up, and tightened on the drainage rod with a nut, channels are formed that taper from about 250µm down to 25µm. One or more of these packs is mounted in a vessel, and the filter may be operated by pumping in the slurry under pressure or, occasionally, by the application of reduced pressure to the outlet side.
In this form, the metafilter can be used as a strainer for coarse particles, but for finer particles a bed of a suitable material such kieselguhr is first built up. The pack of rings, therefore, serves essentially as a base on which the true filter medium is supported.
Advantages
(a) The metafilter possesses considerable strength and high pressures can be used, with no danger of bursting the filter medium.
(b)As there is no filter medium as such, the running costs are low, and it is a very economical
filter.
(c) The metafilter can be made from materials that can provide excellent resistance to corrosion and avoid contamination of the most sensitive product.
(d) by selection of a suitable grade of material to form the bed, it is possible to filter off very fine particles; in fact, it is claimed that some grade will sterilize some liquid by filteration. Equally well it is possible to remove larger particles simply by building up a bed of coarse substances, or even by using the meta filter candle itself if the particles are sufficiently large.
(e) Removal of the cake is effectively carried out by back flushing with water. If further cleaning is required, it is normally necessary to do more than slacken the clamping nut on the end of the drainage rod on which the rings are packed.
Fig:10 (a) surface view ring ,
(b) section through filter
USES OF THE METAFILTER:
The small surface of the metafilter restricts the amount of the solids that can be collected. This, together with the ability to separate very fine particles, means that the metafilter is used almost exclusively for clarification purposes.
Furthermore, the strength of the metafilter permits the use of high pressures (15 bars) making the method suitable for viscous liquids. Also, it can be constructed with material appropriate for corrosive materials. Specific examples of pharmaceutical uses include the clarification os syrups, of injection solutions, and of products such as insulin liquors.
CONCLUSION:
Filtration is an unique unit operation. The seperative process of filtration is widely used in the biopharmaceutical industry to remove contaminants from liquids, air, and gases, such as particulate matter, micro organisms. So a thorough knowledge of filtration equipment and their integrity testing is essential.
References:
- Cooper and Gunn’s. Tutorial Pharmacy by S.J.Carter.
- Pharmaceutical engineering; K. Sambamurthy
- Pharmaceutical engineering; principles and practices, C.V.S. Subrahmanyam
- Encyclopedia of pharmaceutical technology, vol 3, edited by James Swarbrick.
- Pikal, M.J.; Lukes, A.L.; Lang, J.E. Thermal decomposition of amorphous beta-lactam antibacterials. J. Pharm. Sci. 1977, 66, 1312–1316.
- Pikal, M.J.; Lukes, A.L.; Lang, J.E.; Gaines, K. Quantitative crystallinity determinations of beta-lactam antibiotics by solution calorimetry: correlations with stability. J. Pharm. Sci. 1978, 67, 767–773.
- Pikal, M.J.; Dellerman, K.M. Stability testing of pharmaceuticals by high-sensitivity isothermal calorimetry at 25_C: cephalosporins in the solid and aqueous solution states. Int. J. Pharm. 1989, 50, 233–252
- batch and continuous filtration,pharmaceutical filtration ppt, factors affecting rate of filtration, filtration ppt presentation, theory of filtration ppt, advantages and disadvantages of filtration of water,
rate of filtration calculation, filtration techniques ppt, types filtration equipment, filtration equipment pdf,
filtration equipment ppt, simple filtration equipment, filtration equipment chemistry, types of filtration process, types of water filtration, types of filtration pdf.
Filtration equipment pdf
filtration equipment pdf,PDF PPT DOC Pharmaceutical FILTRATION EQUIPMENT – Filtration Mechanism & Types – Adv Disadvantages
[PDF] FILTER INTEGRITY TESTING – FDA Guideline on Sterile Drug Products DOC PPT
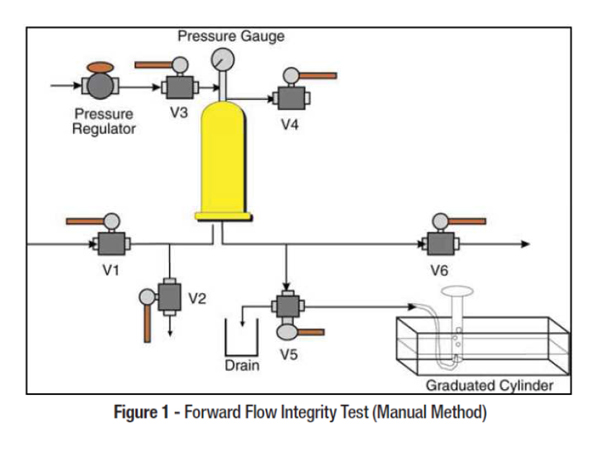
A filter integrity test is a critical unit operation commonly employed in the Pharma industry. FDA Guideline on Sterile Drug Products @ FILTER INTEGRITY TESTING is given below.
FILTER INTEGRITY TESTING
Sterilizing grade filters require testing to assure the filters are integral and fulfill their purpose. Such filter tests are called integrity tests and are performed before and after the filtration process. Sterilizing grade filtration would not be admitted to a process if the filter would not be integrity tested in the course of the process. This fact is also established in several guidelines, recommending the use of integrity testing, pre- and post-filtration. This is not only valid for liquid but also for air filters.
Examples of such guidelines are :
-
FDA Guideline on Sterile Drug Products Produced by Aseptic Processing (1987):
Normally, integrity testing of the filter is performed after the filter unit is assembled and prior to use. More importantly however, such testing should be conducted after the filter is used in order to detect any filter leaks or perforations that may have occurred during filtration.
- The Guide to Inspections of High Purity Water Systems, Guide to Inspections of Lyophilization of Parenterals, and also the CGMP document 212.721 Filters state the following:
- The integrity of all air filters shall be verified upon installation and maintained throughout use. A written testing program adequate to monitor integrity of filters shall be established and followed. Results shall be recorded and maintained as specified in 212.83.
- Solution filters shall be sterilized and installed aseptically. The integrity of solution filters shall be verified by an appropriate test, both prior to any large-volume parenteral solution filtering operation and at the conclusion of such operation before the filters are discarded. If the filter assembly fails the test at the conclusion of the filtering operation, all materials filtered through it during that filtering operation should be rejected. Rejected materials may be refiltered using filters whose integrity has been verified provided that the additional time required for refiltration does not result in a total process time that exceeds the limitations specified in 212.111. Results of each test shall be recorded and maintained as required in 212.188(a).
- ISO 13408-1 First Edition, 1998-08-1, Aseptic Processing of Health Care Products, Part 1: General requirements: Section 17.11.1 Investigation, m. pre- and post-filter integrity test data, and/or filter housing assembly:
- 20.3.1. A validated physical integrity test of a process filter shall be conducted after use without disturbing the filter housing assembly. Filter manufacturer’s testing instructions or recommendations may be used as a basis for a validated method. Physical integrity testing of a process filter should be conducted before use where process conditions permit. ‘‘Diffusive Flow,’’ ‘‘Pressure Hold,’’ and ‘‘Bubble Point’’ are acceptable physical integrity tests.
- 20.3.2. The ability of the filter or housing to maintain integrity in response to sterilization and gas or liquid flow (including pressure surges and flow variations) shall be determined.
- USP 23, 1995, P. 1979. Guide to Good Pharmaceutical manufacturing Practice (Orange
FDA Guide, U.K., 1983):
- PDA (Parenteral Drug Association), Technical Report No. 26, Sterilizing Filtration of Liquids (March 1998):
Integrity tests, such as the diffusive flow, pressure hold, bubble point, or water intrusion tests, are non-destructive tests, which are correlated to the destructive bacteria challenge test with 107/cm2 B. diminuta. Derived from these challenge tests, specific integrity test limits are established, which are described and documented within the filter manufacturers’ literature. The limits are water-based; i.e., the integrity test correlations are performed using water as a wetting medium. If a different wetting fluid, such as a filter or membrane configuration, is used, the integrity test limits may vary. Integrity test measurements depend on the surface area of the filter, the polymer of the membrane, the wetting fluid, the pore size of the membrane, and the gas used to perform the test.
Wetting fluids may have different surface tensions, which can depress or elevate the bubble point pressure. The use of different test gases may elevate the diffusive gas flow. Therefore, appropriate filter validation has to be established to determine the appropriate integrity test limits for the individual process. Bubble Point Test Microporous membranes will fill their pores with wetting fluids by imbibing that fluid in accordance with the laws of capillary rise. The retained fluid can be forced from the filter pores by air pressure applied
from the upstream side. The pressure is increased gradually in increments. At a certain pressure level, liquid will be forced first from the set of largest pores, in keeping with the inverse relationship of the applied air pressure P and the diameter of the pore, d, described in the bubble point equation:
where g is the surface tension of the fluid, y is the wetting angle, P is the upstream pressure at which the largest pore will be freed of liquid, and d is the diameter of the largest pore.
When the wetting fluid is expelled from the largest pore, a bulk gas flow will be detected on the downstream side of the filter system (Fig. 7). The bubble point measurement determines the pore size of the filter membrane, i.e., the larger the pore the lower the bubble point pressure. Therefore, filter manufacturers specify the bubble point limits as the minimum allowable bubble point. During an integrity test, the bubble point test has to exceed the set minimum bubble point.
Manual bubble point test set up
1.Diffusion Test
A completely wetted filter membrane provides a liquid layer across which, when a differential pressure is applied, the diffusive airflow occurs in accordance with Fick’s law of diffusion. This pressure is called test pressure and commonly specified at 80% of the bubble point pressure. In an experimental elucidation of the factors involved in the process, Reti simplified the integrated form of Fick’s law to read as follows:
where N is the permeation rate (moles of gas per unit time), D is the diffusivity of the gas in the liquid, H is the solubility coefficient of the gas, L is the thickness of liquid in the membrane (equal to the membrane thickness if the membrane pores are completely filled
with liquid), P (p1 _ p2) is the differential pressure, and r is the void volume of the membrane, its membrane porosity, commonly around 80%. The size of pores only enter indirectly into the equation; in their combination, they comprise L, the thickness of the liquid layer, the membrane being some 80% porous. The critical measurement of a flaw is the thickness of the liquid layer. Therefore, a flaw or an oversized pore would be measured by the thinning of the liquid layer due to the elevated test pressure on the upstream side. The pore or defect may not be large enough that the bubble point comes into effect, but the
test pressure thins the liquid layer enough to result into an elevated gas flow. Therefore, filter manufacturers specify the diffusive flow integrity test limits as maximum allowable diffusion value. The larger the flaw or a combination of flaw, the higher the diffusive flow.
Pressure Hold Test:
The pressure hold test is a variant of the diffusive airflow test. The test set-up is arranged as in the diffusion test except that when the stipulated applied pressure is reached, the pressure source is valved off. The decay of pressure within the holder is then observed as a function of time, using a precision pressure gauge or pressure transducer.
The decrease in pressure can come from two sources:
1) the diffusive loss across the wetted filter. Because the upstream side pressure in the holder is constant, it decreases progressively as all the while diffusion takes place through the wetted membrane and
2) the source of pressure decay could be a leak of the filter system set-up. An important influence on the measurement of the pressure hold test is the upstream air volume within the filter system. This volume has to be determined first to specify the maximum allowable pressure drop value. The larger the upstream volume, the lower will the pressure drop be. The smaller the upstream volume, the larger the pressure drop. This also means an increase in the sensitivity of the test, and also an increase of temperature influences, if changes occur. Filter manufacturers specify maximum allowable pressure drop values.
2.Water Intrusion Test:
The water intrusion test is used for hydrophobic ventand air membrane filters only. The upstream side of the hydrophobic filter cartridge housing is flooded with water. The water will not flow through the hydrophobic membrane. Air or nitrogen gas pressure is then applied to the upstream side of the filter housing above the water level to a defined test pressure. This is done by way of an automatic integrity tester. A period of pressure stabilization takes place over time frame, by the filter manufacturer’s recommendation, during which the cartridge pleats adjust their positions under imposed pressures.
After the pressure drop thus occasioned stabilizes, the test time starts, and any further pressure drop in the upstream pressurized gas volume, as measured by the automatic tester, signifies a beginning of water intrusion into the largest (hydrophobic) pores, water being incompressible. The automated integrity tester is sensitive enough to detect the pressure drop. This measured pressure drop is converted into a measured intrusion value, which is compared to a set intrusion limit, which has been correlated to the bacteria challenge test. As with the diffusive flow test, filter manufacturers specify a maximum allowable water intrusion value. Above this value, a hydrophobic membrane filter is classified as non-integral.
References for FILTER INTEGRITY TESTING:
- Cooper and Gunn’s. Tutorial Pharmacy by S.J.Carter.
- Pharmaceutical engineering; K. Sambamurthy
- Pharmaceutical engineering; principles and practices, C.V.S. Subrahmanyam
- Encyclopedia of pharmaceutical technology, vol 3, edited by James Swarbrick.
- Pikal, M.J.; Lukes, A.L.; Lang, J.E. Thermal decomposition of amorphous beta-lactam antibacterials. J. Pharm. Sci. 1977, 66, 1312–1316.
- Pikal, M.J.; Lukes, A.L.; Lang, J.E.; Gaines, K. Quantitative crystallinity determinations of beta-lactam antibiotics by solution calorimetry: correlations with stability. J. Pharm. Sci. 1978, 67, 767–773.
Pikal, M.J.; Dellerman, K.M. Stability testing of pharmaceuticals by high-sensitivity isothermal calorimetry at 25_C: cephalosporins in the solid and aqueous solution states. Int. J. Pharm. 1989, 50, 233–252.
FILTER INTEGRITY TESTING PDF – FDA Guideline on Sterile Drug Products PDF
FILTER INTEGRITY TESTING DOC – FDA Guideline on Sterile Drug Products
FILTER INTEGRITY TESTING PPT
FILTER INTEGRITY TESTING – FDA Guideline on Sterile Drug Products is helpful we hope. If you have anything to this please write to us.