NIPER booster 4
1. Identify the wrong statement about Tragacanth
a. Water soluble portion of Tragacanth is called Tragacanthin
b. Water insoluble portion is called Bassorin
c. Best grades of Tragacanth contain high amount of Tragacanthin
d. The source of Indian Tragacanth is Sterculia urens.
2. Identify the correct statement about Hypericin
a. Anthraquinone found in St. Anthony’s fire
b. Phloroglucinol derivative found in St. John’s wort
c. Flavanone glycoside found in St. Anthony’s fire
d. Anthraquinone found in St. John’s wort
3. The first biflavonoid to be isolated was
a. Scutallerin
b. Gingetin
c. Amentoflavone
d. Casticin
4. Dinoprostone is a
a.Synthetic preparation of PGE4α
b. Semi-synthetic preparation of PGF2α
c. Synthetic preparation of PGE2
D. Synthetic preparation of PGE3
5. The 50th element in the periodic table is
a. Fermium
b. Tin
c. Mendleevium
d. Argon
6. The source of Bleomycins is
a. Streptococcus verticillius
b. Staphylococcus faradiae
c. Bleomyces unimyces
d. Bleomyces thermophillus
7. The pink colour of calamine is due to
a. The presence of Ferric chloride
b. The presence of Ferric oxide
c. The presence of Titanium dioxide
d. The presence of Ferrous oxide
8. Plasma Impulse Chemical Vapor Deposition is
a. Method of testing the stability of Type II glass containers
b. Method of coating tablets for extended release
c. Method of coating Type I glass vials
d. Method of testing the alkali resistance of Type III glass containers
9. Hirsutism and Gum hypertrophy are the two prominent adverse effects of
a. Carbamazepine
b. Phenytoin
c. Ethosuximide
d. Lamotrigine
10. Identify the correct combination for obtaining optimal synergy in case of Cotrimoxazole
a. Dose ratio of 20:1 and Plasma concentration 5:1 ( Sulfamethoxazole : Trimethoprim)
b. Dose ratio of 3:2 and Plasm concentration of 20:1 ( Trimethoprim : Sulfamethoxazole)
c. Dose ratio of 5:1 and Plasma concentration of 20:1 ( Sulfamethoxazole : Trimethoprim)
d. Dose ratio of 20:1 and Plasma concentration of 5:1 ( Trimethoprim : Sulfamethoxazole)
ANSWERS on the next page here
NIPER bonus pack 1
Mnemonics for Blood coagulation factors
Mnemonic for remembering Blood coagulation factors
1. Remember factor XIII is Fibrin Stabilizing factor
2. For the remaining 11 factors, memorize this sentence:
Firdous, Prothima ,Tina Called Lalith ,Proveen, Anthony ,Chris and Stuart to Play and Have fun
3. You can decode the sentence by remembering that some of the starting letters indicate the name of a particular factor as given below in bold.
Firdous = Fibrinogen = Factor I
Prothima = Prothrombin = Factor II
Tina = Tissue factor or Thrombolplastin = Factor III
Called = Calcium ions = Factor IV
Lalith = Labile factor or Proaccelerin = Factor V
There is no factor VI !!!
Proveen = Proconvertin = factor VII ( To remove the confusion remember factor V as Labile factor and factor VII as Proconvertin)
Anthony = Antihemophilic factor = factor VIII
Chris = Chirstmas factor = factor IX
Stuart = Stuart factor = factor X
Play = PTA= Plasma Thromboplastin Antecedent
Have fun = Hageman’s factor
This code may not be up to the mark but definitely helps to memorize the factors. If anyone has a better modification then please share with Team Pharmawiki!!!
NIPER booster 3
Niper Booster 3
1. The Director General of World Health Organization is
a. Dr. Ala Alawan
b. Dr. Mirta Roses
c. Dr. Margaret Chan
d. Dr. Shin Young-Soo
2. The New Molecular Entity approved by the FDA for treatment of head lice infections
a. ARGATROBAN
b. SPINOSAD
c. VILAZODONE HYDROCHLORIDE
d. ROFLUMILAST
3. The total number of monographs in the latest edition of Indian Pharmacopoeia
a. 1846
b.1918
c.2340
d.1790
4. How many pints make a gallon?
a. 16
b.20
c.6
d.8
5. Inulin is a
a. Lignan
b. Fructan
c. Flavonoid
d. Resin
6. Borax reaction and Bromine test are
a. Specific tests for Anthraquinone glycosides
b. General tests for Flavonoids
c. Specific test for Tannins
d. General tests for Aloes
7. Identify the Motilin agonist
a. Paclitaxel
b. Ceftrioxime
c. Rifampicin
d. Erythromycin
ANSWERS:
1.c. Dr. Margaret Chan
2.b. SPINOSAD
3.b.1918
4.d.8
5.b. Fructan ( Refer Trease and Evans page 207)
6.d. General tests for Aloes ( Refer Trease and Evans Page 247)
7.d. Erythromycin ( Refer Goodman and Gilman page 636)
NIPER booster 2
Blood & Immunity
Questions will be visible only to registered users. If already registered login here to view the contents below. Press refresh after login
1. How many blood coagulation factors are known?
a. 11
b. 12
c.13
d.14
2. Blood coagulation factor IX is
a. Calcium ions
b. Proaccelerin
c. Hageman’s factor
d. Christmas factor
3. Which one of the following blood coagulation factors does not exist?
a. Factor V
b. Factor VI
c. Factor IV
d. Factor VIII
4. The percentage of blood volume occupied by RBC is known as
a. Differential Blood Count
b. Hematocrit
c. Differential erythrocyte count
d. None of the above
5. Identify the wrong statement about intrinsic pathway of blood coagulation
i. The factors responsible for the onset of this pathway are present within the blood
ii. It occurs rapidly within seconds
iii. It ends with the conversion of fibrinogen to fibrin
iv. The first step in this pathway is the activation of platelets as they come in contact with the exposed collagen fibers of damaged endothelial cells
a. i and iii b. iii and iv c. only ii d. ii and iii
6. Which of the following is/are not an example of MALT
i. Tonsils
ii. Peyer’s patches
iii. Red pulp of spleen
iv. Nodes of Ranvier
a. i b. ii c. i and iv d. iii and iv
7. A typical ECG shows the following
i. P wave, QRS complex, T wave, PQ segment, QT segment, ST interval
ii. P wave, QRS complex, T elevation, PQ interval, QT interval and ST segment
iii. P wave, QRS complex, T wave, PQ interval, QT interval and ST segment
iv. P elevation, QRS wave, T elevation, PQ segment, QT interval and ST interval
8. The injectable formulation E-mal, marketed in India by Themis medicare, Mumbai contains a new drug which was developed by one of the following research institutes in India
a. CIMAP – Lucknow
b. CDRI – Lucknow
c. NIPER – S.A.S Nagar
d. NCL – Pune
9. The headquarters of WIPO are located at
a. Geneva, Switzerland
b. Copenhagen, Denmark
c. Belfast, Ireland
d. Washington D.C, USA
10. Identify the heterocyclic ring(s) present in Fluoxetine
i. Quinoxaline
ii. Quinazoline
iii. Morpholine
iv. None of the above
Answers:
1.a
2. d. Christmas factor
3. b. Factor VI
4. b. Hematocrit
5. d. ii and iii
6. d. iii and iv
7. iii. P wave, QRS complex, T wave, PQ interval, QT interval and ST segment
8. b. CDRI – Lucknow
9. a. Geneva, Switzerland
10. iv. None of the above
FDA issues final rule on sterility testing of biological products
FDA issued a final rule on sterility testing on May 3, 2012, that amends the requirements for most licensed biological products and aims to provide manufacturers with the flexibility, as appropriate, to keep pace with technological and scientific advances. The rule is in response to President Obama’s Executive Order 13563 which called for improving regulation and regulatory review.
Specifically, the rule revises sterility requirements under Title 21 of the Code of Federal Regulations (CFR), subchapter F, parts 600 through 680 as follows:
- “Eliminates specified sterility test methods, culture media formulae (or formulation), and culture media test requirements
- Eliminates specified membrane filtration procedure requirements for certain products
- Eliminates specified sterility test requirements for most bulk material
- Modifies the repeat sterility test requirements, so that repeat tests will occur only once for each lot
- Replaces the storage and maintenance requirements for cultures of test organisms used to determine the “growth-promoting qualities” of culture media with validation requirements specifying that any sterility test used is able to consistently detect the presence of viable contaminating microorganisms, and with verification of “growth-promoting properties” or microorganism-detection capabilities of test and test components
- Replaces the sample size or amount requirement with a requirement that the sample be appropriate to the material being tested
- Replaces the Interpretation of test results section under § 610.12(c) with a requirement that manufacturers establish, implement, and follow written procedures for sterility testing that describe, at a minimum, the test method used, the method of sampling, and the written specifications for acceptance or rejection of each lot
- Simplifies and clarifies the Exceptions section under § 610.12(h)
- Identifies the Director of CDER as one of the two Center directors authorized to grant an exemption under the exception provision at § 610.12(h)(2). In the proposed rule, the Center for Devices and Radiological Health was erroneously identified in this exception, instead of the Center for Drug Evaluation and Research
- Revises the definition of the term “sterility” under § 600.3(q).
- Eliminates certain exceptions for allergenic products related to sterility testing under § 680.3(c).”
Companies will not have long to implement the changes as the rule takes effect June 4, 2012. The proposed rule received several comments from industry and the final rule includes the agency’s response to those recommendations. Overall, FDA “recognizes the role innovation plays in bringing safe and effective products to market in a timely and cost-efficient manner,” according to an FDA announcement of the rule. “This action reflects the agency’s efforts to review and, as necessary, update biologics regulations, to keep pace with technological developments and to boost regulatory science.”
More Info Click Here
Source:
NIPER booster 1
General Knowledge –
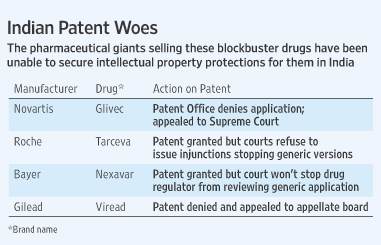
1. The Drug of Novartis that is challenged in the Indian courts for the validity of Patent?
2. Fundamental rights of an Indian Citizen are ____ in number.
3. Lipitor is the brand name of which drug?
4.Pharmaceutical Patents in India are currently granted as —————— patents (product/process)
5. The Director General of World Health Organization is
a. Dr. Ala Alawan
b. Dr. Mirta Roses
c. Dr. Margaret Chan
d. Dr. Shin Young-Soo
NOTE: This is a trial pack with only 5 questions and explanations. You can expect more questions every day. We have a lot of mnemonics that will help you for your NIPER preparation. You can also send your questions by using the contact form above. From tomorrow onwards we will provide the NIPER booster packs only to registered users, so please register by clicking here
Answers:
1. Gleevec, Imitinab mesylate, is used in treating chronic myelogenous leukemia (CML), gastrointestinal stromal tumors (GISTs) and some other diseases. It was challenged in the MADRAS HIGH COURT on the grounds lacking NOVELTY as per Section 3(d) of Indian Patent Act. Gleevec was said to lack novelty because it was just a different salt form with no improved therapeutic efficacy of an already existing patent. (a term called EVERGREENING of patents)
http://en.wikipedia.org/wiki/Imatinib
2. The six fundamental rights recognised by the constitution are:
1) Right to equality, including equality before law, prohibition of discrimination on grounds of religion, race, caste, sex or place of birth, and equality of opportunity in matters of employment, abolition of untouchability and abolition of titles.
2) Right to freedom which includes speech and expression, assembly, association or union or cooperatives, movement, residence, and right to practice any profession or occupation (some of these rights are subject to security of the State, friendly relations with foreign countries, public order, decency or morality), right to life and liberty, right to education, protection in respect to conviction in offences and protection against arrest and detention in certain cases.
3) Right against exploitation, prohibiting all forms of forced labour, child labour and traffic in human beings;
4) Right to freedom of religion, including freedom of conscience and free profession, practice, and propagation of religion, freedom to manage religious affairs, freedom from certain taxes and freedom from religious instructions in certain educational institutes.
5) Cultural and Educational rights preserving Right of any section of citizens to conserve their culture, language or script, and right of minorities to establish and administer educational institutions of their choice; and
6) Right to constitutional remedies for enforcement of Fundamental Rights.
http://en.wikipedia.org/wiki/Fundamental_Rights_in_India#Right_To_Education
3. Lipitor, is trade name of the calcium salt of Atorvastatin marketed by Pfizer . It is a member of the drug class known as statins, used for lowering blood cholesterol. Generic atorvastatin, manufactured by generic drugmakers Watson Pharmaceuticals and India’sRanbaxy Laboratories, began being available in the United States on November 30, 2011 and are going to be cheaper than Lipitor.
http://en.wikipedia.org/wiki/Atorvastatin
4. Pharmaceutical patents in India are granted as PRODUCT patents as per Indian Patent Law of 2005. earlier they were granted process patents.However Until 2005, Indian law recognized only process patents for making pharmaceutical products—and not the actual products.
http://online.wsj.com/article/SB10001424052748703455804575057621354459804.html
5. Dr. Margaret Chan
NIPER Daily Booster Pack
NIPER Daily Booster pack…. Starting 9th May 2012
Every day we will put 10-15 questions from various sources that are quite essential for NIPER-JEE aspirants.
Get high impact questions with explanations everyday…
Highly useful for NIPER-JEE aspirants…
Register on the site to GET updates…
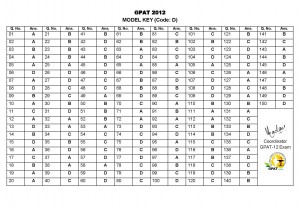
GPAT 2012 Official Key – Series D
Download the official Key for GPAT 2012 for Paper code D
Download as PDF ===> GPAT_2012_Key_Code_D
Download as JPG
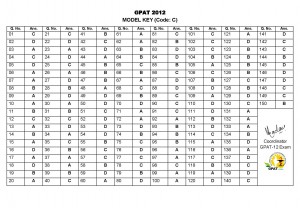
GPAT 2012 Official Key – Series C
Download the official Key for GPAT 2012 for Paper code C
Download as PDF ===> GPAT_2012_Key_Code_C
Download as JPG